Abstract
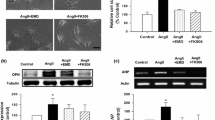
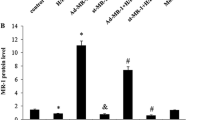
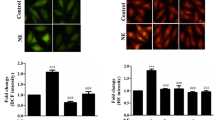
Increased osteopontin (OPN) expression in the heart, specifically in myocytes, associates with increased myocyte apoptosis and myocardial dysfunction. Recently, we provided evidence that OPN interacts with CD44 receptor, and induces myocyte apoptosis via the involvement of endoplasmic reticulum stress and mitochondrial death pathways. Here we tested the hypothesis that OPN induces oxidative stress in myocytes and the heart via the involvement of mitochondria and NADPH oxidase-4 (NOX-4). Treatment of adult rat ventricular myocytes (ARVMs) with OPN (20 nM) increased oxidative stress as analyzed by protein carbonylation, and intracellular reactive oxygen species (ROS) levels as analyzed by ROS detection kit and dichlorohydrofluorescein diacetate staining. Pretreatment with NAC (antioxidant), apocynin (NOX inhibitor), MnTBAP (superoxide dismutase mimetic), and mitochondrial KATP channel blockers (glibenclamide and 5-hydroxydecanoate) decreased OPN-stimulated ROS production, cytosolic cytochrome c levels, and apoptosis. OPN increased NOX-4 expression, while decreasing SOD-2 expression. OPN decreased mitochondrial membrane potential as measured by JC-1 staining, and induced mitochondrial abnormalities including swelling and reorganization of cristae as observed using transmission electron microscopy. OPN increased expression of BIK, a pro-apoptotic protein involved in reorganization of mitochondrial cristae. Expression of dominant-negative BIK decreased OPN-stimulated apoptosis. In vivo, OPN expression in cardiac myocyte-specific manner associated with increased protein carbonylation, and expression of NOX-4 and BIK. Thus, OPN induces oxidative stress via the involvement of mitochondria and NOX-4. It may affect mitochondrial morphology and integrity, at least in part, via the involvement of BIK.








Similar content being viewed by others
Abbreviations
- OPN:
-
Osteopontin
- ARVMs:
-
Adult rat ventricular myocytes
- ROS:
-
Reactive oxygen species
- NADPH oxidase:
-
Nicotinamide adenine dinucleotide phosphate-oxidase
- NOX:
-
NADPH oxidase
- NAC:
-
N-acetyl-l-cysteine
- SOD-2:
-
Mitochondrial superoxide dismutase-2
- DMEM:
-
Dulbecco’s modified eagle medium
- APO:
-
Apocynin
- GLIB:
-
Glibenclamide
- 5-HD:
-
5-hydroxydecanoate
- H2DCFDA:
-
2′,7′-dichlorohydrofluorescein diacetate
- mitoKATP :
-
Mitochondrial KATP channels
- MnTBAP:
-
Manganese (III) tetrakis (4-benzoic acid) porphyrin
- CTL:
-
Control
- MAM:
-
Mitochondrial-associated endoplasmic reticulum membrane
- MHC-OPN:
-
OPN transgenic mice
- Mut BIK:
-
Mutant BIK
- ER:
-
Endoplasmic reticulum
References
Frangogiannis NG (2012) Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92:635–688
Singh M, Foster CR, Dalal S, Singh K (2010) Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 48:538–543
Singh M, Dalal S, Singh K (2014) Osteopontin: at the cross-roads of myocyte survival and myocardial function. Life Sci 118:1–6
Wang KX, Denhardt DT (2008) Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev 19:333–345
Denhardt DT, Noda M, O’Regan AW et al (2001) Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
Kahles F, Findeisen HM, Bruemmer D (2014) Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab 3:384–393
Matsui Y, Jia N, Okamoto H et al (2004) Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 43:1195–1201
Satoh M, Nakamura M, Akatsu T et al (2005) Myocardial osteopontin expression is associated with collagen fibrillogenesis in human dilated cardiomyopathy. Eur J Heart Fail 7:755–762
Subramanian V, Krishnamurthy P, Singh K, Singh M (2007) Lack of osteopontin improves cardiac function in streptozotocin-induced diabetic mice. Am J Physiol Heart Circ Physiol 292:H673–H683
Sam F, Xie Z, Ooi H et al (2004) Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens 17:188–193
Renault MA, Robbesyn F, Reant P et al (2010) Osteopontin expression in cardiomyocytes induces dilated cardiomyopathy. Circ Heart Fail 3:431–439
Dalal S, Zha Q, Daniels CR et al (2014) Osteopontin stimulates apoptosis in adult cardiac myocytes via the involvement of CD44 receptors, mitochondrial death pathway, and endoplasmic reticulum stress. Am J Physiol Heart Circ Physiol 306:H1182–H1191
Sugamura K, Keaney JF (2011) Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51:978–992
Sun L, Fan H, Yang L et al (2015) Tyrosol prevents ischemia/reperfusion-induced cardiac injury in H9c2 cells: involvement of ROS, Hsp70, JNK and ERK, and apoptosis. Molecules 20:3758–3775
Vichova T, Motovska Z (2013) Oxidative stress: predictive marker for coronary artery disease. Exp Clin Cardiol 18:e88–e91
Ago T, Kuroda J, Pain J et al (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106:1253–1264
Weiss JN, Korge P, Honda HM, Ping P (2003) Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93:292–301
Baines CP, Kaiser RA, Purcell NH et al (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662
Kuroda J, Ago T, Matsushima S et al (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107:15565–15570
Bravo-Sagua R, Rodriguez AE, Kuzmicic J et al (2013) Cell death and survival through the endoplasmic reticulum-mitochondrial axis. Curr Mol Med 13:317–329
Germain M, Mathai JP, McBride HM, Shore GC (2005) Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 24:1546–1556
Germain M, Mathai JP, Shore GC (2002) BH-3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J Biol Chem 277:18053–18060
Hegde R, Srinivasula SM, Ahmad M et al (1998) Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist. J Biol Chem 273:7783–7786
Germain M, Mathai JP, McBride HM, Shore GC (2005) Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 24:1546–1556
Coultas L, Bouillet P, Stanley EG et al (2004) Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol 24:1570–1581
Irita J, Okura T, Jotoku M et al (2011) Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Ren Physiol 301:F833–F844
Wolak T, Kim H, Ren Y et al (2009) Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int 76:32–43
Deka S, Vanover J, Sun J et al (2007) An early event in the herpes simplex virus type-2 replication cycle is sufficient to induce Chlamydia trachomatis persistence. Cell Microbiol 9:725–737
Dalle-Donne I, Aldini G, Carini M et al (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406
Luk E, Carroll M, Baker M, Culotta VC (2003) Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc Natl Acad Sci USA 100:10353–10357
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519
Lai C-F, Seshadri V, Huang K et al (2006) An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res 98:1479–1489
Noma A (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305:147–148
Holmuhamedov EL, Jovanović S, Dzeja PP et al (1998) Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol 275:H1567–H1576
Hu H, Sato T, Seharaseyon J et al (1999) Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels. Mol Pharmacol 55:1000–1005
Garlid KD, Paucek P, Yarov-Yarovoy V et al (1996) The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem 271:8796–8799
Nolly MB, Caldiz CI, Yeves AM et al (2014) The signaling pathway for aldosterone-induced mitochondrial production of superoxide anion in the myocardium. J Mol Cell Cardiol 67:60–68
Zhang DX, Chen YF, Campbell WB et al (2001) Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res 89:1177–1183
Eskes R, Desagher S, Antonsson B, Martinou JC (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20:929–935
Zong WX, Li C, Hatzivassiliou G et al (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162:59–69
Han J, Sabbatini P, White E (1996) Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol Cell Biol 16:5857–5864
Chinnadurai G, Vijayalingam S, Rashmi R (2008) BIK, the founding member of the BH3-only family proteins: mechanisms of cell death and role in cancer and pathogenic processes. Oncogene 27(Suppl 1):S20–S29
Acknowledgments
The authors appreciate the technical help received from Dr. Dennis Defoe, Dr. Robert V. Schoborg, Mr. Rolf Fritz, Ms. Jennifer Kintner, and Ms. Barbara A. Connelly.
Funding
This work was supported by Merit Review awards (BX002332 and BX000640) from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development, National Institutes of Health (R15HL129140), and funds from Institutional Research and Improvement account (to KS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Rights and permissions
About this article
Cite this article
Dalal, S., Zha, Q., Singh, M. et al. Osteopontin-stimulated apoptosis in cardiac myocytes involves oxidative stress and mitochondrial death pathway: role of a pro-apoptotic protein BIK. Mol Cell Biochem 418, 1–11 (2016). https://doi.org/10.1007/s11010-016-2725-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2725-y