Abstract
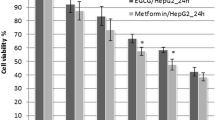
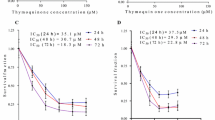
Hepatocellular carcinoma is one of the most common lethal diseases worldwide and there is no effective treatment till date. Natural products derived from the plants play an important role in chemoprevention and act as therapeutic antitumor agents. Licorice is a plant that has been used in food and medicine for the treatment of various diseases. 18β-Glycyrrhetinic acid (18β-GA), a pentacyclic triterpenoid obtained from the roots of licorice plant, is reported to possess various pharmacological properties such as antitumor and antiinflammatory activities. The present study was designed to elucidate the chemopreventive effect of 18β-GA through antiinflammation, antiproliferation, and induction of apoptosis in human hepatoma cell line HepG2. 18β-GA significantly inhibits the proliferation of HepG2 cell without affecting the normal liver cell line (Chang’s). In the present study, 18β-GA increased the formation of reactive oxygen species, nitric oxide production, and loss of mitochondrial membrane potential, suggesting the involvement of 18β-GA in apoptosis which was also confirmed by assessing the markers involved in apoptosis like caspase-3, caspase-9, Bax:Bcl-2 ratio, and cleaved PARP. 18β-GA also downregulated the expression of inflammatory proteins such as NF-κB, iNOS, and COX-2. Keeping these data into consideration, our results suggest that 18β-GA may be used as a chemopreventive agent in liver cancer.







Similar content being viewed by others
References
Irfan A, Dileep NL (2006) Malignant tumours of liver. Surgery 25:34–41
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:5–16
Sheu JC (1997) Molecular mechanism of hepatocarcinogenesis. J Gastroenterol Hepatol 12:S309–S313
Tsai JF, Chang WY, Jeng JE, Ho MS, Lin ZY, Tsai JH (1994) Hepatitis B and C virus infection as risk factors for liver cirrhosis and cirrhotic hepatocellular carcinoma: a case-control study. Liver 14:98–102
Jackson PE, Groopman JD (1999) Aflatoxin and liver cancer. Baillieres Clin Gastroenterol 13:545–555
Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, Kosuge T, Okada S, Takayasu K, Yamasaki S (1998) Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology 28:1241–1246
Poon RT, Fan ST (2004) Resection prior to liver transplantation for hepatocellular carcinoma: a strategy of optimizing the role of resection and transplantation in cirrhotic patients with preserved liver function. Liver Transpl 10:813–815
Curley SA, Izzo F (2002) Radiofrequency ablation of primary and metastatic liver tumors. Surg Technol Int 10:99–106
Sporn MB, Liby KT (2005) Cancer chemoprevention: scientific promise, clinical uncertainty. Nat Clin Pract Oncol 2:518–525
Wang CY, Kao TC, Lo WH, Yen GC (2011) Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agric Food Chem 59(14):7726–7733
Maitraie D, Hung CF, Tu HY, Liou YT, Wei BL, Yang SC, Wang JP, Lin CN (2009) Synthesis, anti-inflammatory, and antioxidant activities of 18beta-glycyrrhetinic acid derivatives as chemical mediators and xanthine oxidase inhibitors. Bioorg Med Chem 17(7):2785–2792
Nishino H, Yoshioka K, Iwashima A, Takizawa H, Konishi S, Okamoto H, Okabe H, Shibata S, Fujiki H, Sugimura T (1986) Glycyrrhetic acid inhibits tumor-promoting activity of teleocidin and 12-O-tetradecanoylphorbol-13-acetate in two-stage mouse skin carcinogenesis. Jpn J Cancer Res 77(1):33–38
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappaB activation. Mutat Res 480–481:243–268
Hasan S, Khan R, Ali N, Khan A, Rehman M, Tahir M, Lateef A, Nafees S, Mehdi S, Rashid S, Shahid A, Sultana S (2015) 18-β Glycyrrhetinic acid alleviates 2-acetylaminofluorene-induced hepatotoxicity in Wistar rats: role in hyperproliferation, inflammation and oxidative stress. Hum Exp Toxicol 34(6):628–641
Reed JC (1999) Dysregulation of apoptosis in cancer. J Clin Oncol 17:2941–2953
Dupont-Versteegden EE (2005) Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40:473–481
Schwartz LM (2008) Atrophy and programmed cell death of skeletal muscle. Cell Death Differ 15:1163–1169
Fabregat I, Roncero C, Fernández M (2007) Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int 27:155–162
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
Rossi T, Castelli M, Zandomeneghi G, Ruberto A, Benassi L, MagnoniC Santachiara S, Baggio G (2003) Selectivity of action of glycyrrhizin derivatives on the growth of MCF-7 and HEP-2cells. Anticancer Res 23:3813–3818
Hibasami H, Iwase H, Yoshioka K, Takahashi H (2005) Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60cells. Int J Mol Med 16:233–236
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124
Decker T, Lohmann-M ML (1988) A quick and simple method for the quantitation of lactate hydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 15:61–69
Koh JY, Choi DW (1987) Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods 20:83–90
Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Chen LB (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate forming lipophilic cation JC-1. Proc Natl Acad Sci 88:3671–3675
Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol 141:2407–2412
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci 78:431–441
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421
Hasan SK, Sultana S (2015) Geraniol attenuates 2-acetylaminofluorene induced oxidative stress, inflammation and apoptosis in the liver of wistar rats. Toxicol Mech Methods 25(7):559–573
Siddiqi A, Nafees S, Rashid S, Sultana S, Saidullah B (2015) Hesperidin ameliorates trichloroethylene-induced nephrotoxicity by abrogation of oxidative stress and apoptosis in wistar rats. Mol Cell Biochem 406(1–2):9–20
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P (2008) Potential of spice-derived phytochemicals for cancer prevention. Planta Med 74:1560–1569
Shanmugam MK, Kannaiyan R, Sethi G (2011) Targeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancer. Nutr Cancer 63:161–173
Aggarwal BB, Ichikawa H, Garodia P, Weerasinghe P, Sethi G, Bhatt ID, Pandey MK, Shishodia S, Nair MG (2006) From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Exp Opin Ther Targets 10:87–118
Asl MN, Hosseinzadeh H (2008) Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res 22:709–724
Rossi T, Benassi L, Magnoni C, Ruberto AI, Coppi A, Baggio G (2005) Effects of glycyrrhizin on UVB-irradiated melanoma cells. In Vivo 19:319–322
Clemedson C, Ekwall B (1999) Overview of the final MEIC results. The in vitro–in vitro evaluation. Toxicol In Vitro 13:657–663
Scheers ME, Ba Ekwall, Dierickx JP (2001) In vitro long-term cytotoxicity testing of 27 MEIC chemicals on HepG2 cells and comparison with acute human toxicity data. Toxicol In Vitro 15:153–161
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K (2008) Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-B cells. Toxicol Lett 180:222–229
Asharani PV, Low Kah Mun G, Hande MP (2009) Cytotoxicity and genotoxicity of sliver nanoparticles in human cells. ACS Nano 3:279–290
Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, Hussain SM, Hofmann MC (2010) Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci 116:577–589
Foldbjerg R, Dang DA, Autrup H (2011) Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol 85:743–750
Johnson LV, WalshML Chen LB (1980) Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci USA 77:990–994
Zhou L, Zhu DY (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20:223–230
Finocchietto PV, Franco MC, Holod S, Gonzalez AS, Converso DP, Arciuch VG, Serra MP, Poderoso JJ, Carreras MC (2009) Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death. Exp Biol Med 234:1020–1028
Leonelli M, Torrao AS, Britto LR (2009) Unconventional neurotransmitters, neurodegeneration and neuroprotection. Braz J Med Biol Res 42:68–75
Moriya R, Uehara T, Nomura Y (2000) Mechanism of nitric oxide-induced apoptosis in human neuroblastoma SH-SY5Y cells. FEBS Lett 484:253–260
Zargan J, Sajad M, Umar S, Naime M, Ali S, Khan HA (2011) Scorpion (Odontobuthus doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol Cell Biochem 384:173–181
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Iliopoulos D, Hirsch HA, Struhl K (2009) An epigenetic switch involving NF-κB, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell 139(4):693–706
Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ (2002) The role of IKKin constitutive activation of NF-κB transcription factor in prostate carcinoma cells. J Cell Sci 115(1):141–151
Suh J, Payvandi F, Edelstein LC, Amenta PS, ZongW-X Gélinas C (2002) Mechanisms of constitutive NF-κB activation in human prostate cancer cells. Prostate 52(3):183–200
Shankar S, Ganapathy S, Srivastava RK (2008) Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res 14(21):6855–6866
Benjamin CW, Hiebsch RR, Jones DA (1998) Caspase activation in MCF7 cells responding to etoposide treatment. Mol Pharmacol 53:446–450
Ueda N, Shah SV (1994) Apoptosis. J Lab Clin Med 124:169–177
Sharma G, Kar S, Palit S, Das PK (2012) 18β-glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell Physiol 227(5):1923–1931
Lee CS, Kim YJ, Lee MS, Han ES, Lee SJ (2008) 18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci 83:481–489
Acknowledgments
The author S. S. is thankful to the Indian Council of Medical Research (ICMR), New Delhi, India, for providing fellowship to the first author (S. K. H.) to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hasan, S.K., Siddiqi, A., Nafees, S. et al. Chemopreventive effect of 18β-glycyrrhetinic acid via modulation of inflammatory markers and induction of apoptosis in human hepatoma cell line (HepG2). Mol Cell Biochem 416, 169–177 (2016). https://doi.org/10.1007/s11010-016-2705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2705-2