Abstract
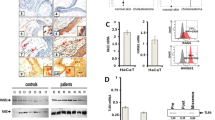
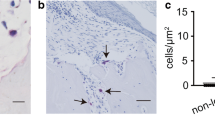
High-mobility group box chromosomal protein 1 (HMGB-1), a nuclear DNA binding protein, was recently rediscovered as a new proinflammatory cytokine. The purpose of this study was to determine HMGB-1 expression in vivo and to identify the effect of extracellular HMGB-1 in inflammatory process associated with bone destruction in cholesteatoma. We investigated the expression and location of HMGB-1 in the cholesteatoma and healthy skin using an immunofluorescence assay. We also detected apoptosis and DNA fragments in the cholesteatoma by TUNEL staining. HMGB-1 concentration in apoptotic supernatants from UV light-treated cells, culture supernatants and its translocation in cholesteatoma keratinocytes stimulated by supernatants from UV light-treated cells were measured by immunoblot analysis and immunofluorescence assay. Cultures of human cholesteatoma keratinocytes were exposed to CpG-DNA, HMGB-1, or CpG-DNA complexed to HMGB-1 for 24 h. Cytokines in the culture supernatant were measured by ELISA. In addition, levels of proinflammatory cytokines released by cholesteatoma keratinocytes stimulated by supernatants from UV light-treated cells with or without anti-HMGB-1 antibodies and supernatants from UV light-treated cells with DNase 1 were measured by enzyme-linked immunosorbent assay. The expression of HMGB-1 in cholesteatoma increased and it translocated both to the cytoplasm and extracellular space. Furthermore, the HMGB-1 concentration in supernatants increased significantly after addition of supernatants from UV light-treated cells. TNF-α and IL-1β can be induced by purified HMGB-1 combined with CpG-DNA in the cholesteatoma keratinocytes. In addition, supernatants of apoptotic cells containing HMGB-1–DNA were effective in inducing TNF-α and IL-1β secretion. This study suggested that persistent expression of extracellular HMGB-1 and DNA fragments in cholesteatoma leads to TNF-α and IL-1β production, causing bone resorption and destruction. Thus, we have implicated that HMGB-1–DNA complexes might act as a key molecule involved in bone resorption associated with cholesteatoma.













Similar content being viewed by others
References
Bujia J, Holly A, Antoli-Candela F, Tapia MG, Kastenbauer E (1996) Immunobiological peculiarities of cholesteatoma in children: quantification of epithelial proliferation by MIB1. Laryngoscope 106:865–868
Chole RA (1997) The molecular biology of bone resorption due to chronic otitis media. Ann N Y Acad Sci 830:95–109
Kurihama A, Toshima M, Yuasa R, Takasaka T (1991) Bone resorption mechanisms in chronic otitis media with cholesteatoma: specific production by cholesteatoma tissue in culture of bone-resorbing activity attributable to interleukin-1 alpha. Ann Otol Rhinol Laryngol 100:989–998
Yan SD, Huang CC (1991) The role of tumor necrosis factor-alpha in bone resorption of cholesteatoma. Am J Otolaryngol 12:83–89
Kinoshita T (1994) The roles of interleukin-1, tumor necrosis factor-alpha and parathyroid hormone in bone resorption of cholesteatoma otitis. Nihon Jibiinkoka Gakkai Kaiho 97:1472–1480
Ahn JM, Huang CC, Abramson M (1990) Localization of interleukin-1 in human cholesteatoma. Am J Otolaryngol 11:71–77
Amar MS, Wishahi HF, Zakhary MM (1996) Clinical and biochemical studies of bone destruction in cholesteatoma. J Laryngol Otol 110:534–539
Akimoto R, Pawankar R, Yagi T, Baba S (2000) Acquired and congenital cholesteatoma: determination of tumor necrosis factor-alpha, intercellular adhesion molecule-1, interleukin-1-alpha and lymphocyte functional antigen-1 in the inflammatory process. ORL J Otorhinolaryngol Relat Spec 62:257–265
Goodwin GH, Sanders C, Johns EW (1973) A new group of chromatin associated proteins with a high content of acidic and basic amino acids. Eur J Biochem 38:14–19
Andersson U, Erlandsson-Harris H, Yang H, Yang H, Tracey KJ (2002) HMGB1 as a DNA-binding cytokine. J Leukoc Biol 72:1084–1091
Wang H, Bloom O, Zhang M et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Bell CW, Jiang W, Reich CF, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291:1318–1325
Andersson U, Wang H, Palmblad K et al (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377
Tian J, Avalos AM, Mao SY et al (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496
Ivanov S, Dragoi AM, Wang X et al (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110:1970–1981
Lövgren T, Eloranta ML, Båve U, Alm GV, Ronnblom L (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50:1861–1872
Shinoda H, Huang CC (1995) Expressions of c-jun and p53 proteins in human middle ear cholesteatoma: relationship to keratinocyte proliferation, differentiation, and programmed cell death. Laryngoscope 105:1232–1237
Olszewska E, Chodynicki S, Chyczewski L (2006) Apoptosis in the pathogenesis of cholesteatoma in adults. Eur Arch Otorhinolaryngol 263:409–413
Yang H, Wang H, Tracey KJ (2001) HMG-1 rediscovered as a cytokine. Shock 15:247–253
Czura CJ, Tracey KJ (2003) Targeting high mobility group box 1 as a late-acting mediator of inflammation. Crit Care Med 31:46–50
Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H (2007) Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J Leukoc Biol 81:49–58
Pisetsky DS, Fairhurst AM (2007) The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 40:281–284
Felix R, Fleisch H, Elford PR (1989) Bone-resorbing cytokines enhance release of macrophage colony stimulating activity by the osteoblastic cell MC3T3-E1. Calcif Tissue Int 44:356–3560
Robinson DR, Tashjian AH Jr, Levine L (1975) Prostaglandin stimulated bone resorption by rheumatoid synovia: a possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest 56:1181–1188
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumor. Proc Natl Acad Sci 72:3666–3670
Acknowledgments
This work was supported by the Shanghai Shen-kang Medical Science Project SHDC12010119, Natural Science Foundation Project of CQ CSTC and Ministry Clinical Disciplines Project 2010-439.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, Z., Wang, Z., Liang, Q. et al. Induction of cytokine production in cholesteatoma keratinocytes by extracellular high-mobility group box chromosomal protein 1 combined with DNA released by apoptotic cholesteatoma keratinocytes. Mol Cell Biochem 400, 189–200 (2015). https://doi.org/10.1007/s11010-014-2275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2275-0