Abstract
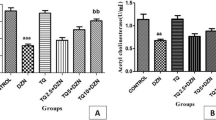
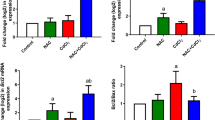
The ex vivo and in vitro effects of quercetin on NTPDase, adenosine deaminase (ADA), and acetycholinesterase (AChE) activities in lymphocytes, as well as the effects of quercetin on butyrylcholinesterase (BChE) activity in serum and myeloperoxidase (MPO) activity in plasma were determined in rats. For the ex vivo experiment, animals were orally exposed to Cadmium (Cd) for 45 days. Animals were divided into eight groups: saline/ethanol, saline/Querc 5 mg/kg, saline/Querc 25 mg/kg, saline/Querc 50 mg/kg, Cd/ethanol, Cd/Querc 5 mg/kg, Cd/Querc 25 mg/kg, and Cd/Querc 50 mg/kg. The ex vivo data showed an increase in the ATP and ADP hydrolysis and ADA activity in Cd-exposed rats when compared to the control group. The treatment with quercetin 25 and 50 mg/kg prevented this increase in the ATP and ADP hydrolysis, while the treatment with quercetin 5, 25, and 50 mg/kg prevented the increase in the ADA activity. AChE, BChE, and MPO activities ex vivo presented an increase in the Cd-exposed group when compared to the control group, and the treatment with quercetin 5, 25, and 50 mg/kg prevented this increase caused by Cd exposure. The in vitro experiment showed that quercetin 5, 10, 25, or 50 µM decreased the ADA activity proportionally to the increase of the concentrations of quercetin when compared to the control group. Thus, we can suggest that the quercetin is able to modulate NTPDase, ADA, AChE, and MPO activities and contribute to maintain the levels of ATP, adenosine, and acetylcholine normal, respectively, exhibiting potent pro-inflammatory and anti-inflammatory actions.




Similar content being viewed by others
References
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich AGroneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:22. doi:10.1186/1745-6673-1-22
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. doi:10.1016/j.taap.2009.04.020
Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M (1998) Health effects of cadmium exposure: a review of the literature and a risk estimate. Scand J Work Environ Health 24(Suppl 1):1–51
Jarup L, Hellstrom L, Alfven T, Carlsson MD, Grubb A, Persson B, Pettersson C, Spang G, Schutz A, Elinder CG (2000) Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 57:668–672
Goncalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60. doi:10.1016/j.cbi.2010.04.011
Lafuente A, Gonzalez-Carracedo A, Romero A, Esquifino AI (2003) Effect of cadmium on lymphocyte subsets distribution in thymus and spleen. J Physiol Biochem 59:43–48
Delves PJ, Roitt IM (2000) The immune system. Second of two parts. N Engl J Med 343:108–117. doi:10.1056/NEJM200007133430207
Rock KL, Hearn A, Chen CJ, Shi Y (2005) Natural endogenous adjuvants. Springer Semin Immunopathol 26:231–246. doi:10.1007/s00281-004-0173-3
Mancino G, Placido RDi, Virgilio F (2001) P2X7 receptors and apoptosis in tuberculosis infection. J Biol Regul Homeost Agents 15:286–293
Kawashima K, Fujii T (2003) The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci 74:675–696
Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112:358–404. doi:10.1016/j.pharmthera.2005.04.013
Maliszewski CR, Delespesse GJ, Schoenborn MA, Armitage RJ, Fanslow WC, Nakajima T, Baker E, Sutherland GR, Poindexter K, Birks C et al (1994) The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol 153:3574–3583
Burch LHPicher M (2006) E-NTPDases in human airways: regulation and relevance for chronic lung diseases. Purinergic Signal 2:399–408. doi:10.1007/s11302-006-9001-7
Linden J (2006) New insights into the regulation of inflammation by adenosine. J Clin Invest 116:1835–1837. doi:10.1172/JCI29125
Zimmermann H (1992) 5′-Nucleotidase: molecular structure and functional aspects. Biochem J 285(Pt 2):345–365
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2:409–430. doi:10.1007/s11302-006-9003-5
Conlon BA, Law WR (2004) Macrophages are a source of extracellular adenosine deaminase-2 during inflammatory responses. Clin Exp Immunol 138:14–20. doi:10.1111/j.1365-2249.2004.02591.x
Schetinger MR, Morsch VM, Bonan CD, Wyse AT (2007) NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. BioFactors 31:77–98
Tayebati SK, El-Assouad D, Ricci A, Amenta F (2002) Immunochemical and immunocytochemical characterization of cholinergic markers in human peripheral blood lymphocytes. J Neuroimmunol 132:147–155
de Almeida JP, Saldanha C (2010) Nonneuronal cholinergic system in human erythrocytes: biological role and clinical relevance. J Membr Biol 234:227–234. doi:10.1007/s00232-010-9250-9
Pavlov V, Xiao Y, Willner I (2005) Inhibition of the acetycholine esterase-stimulated growth of Au nanoparticles: nanotechnology-based sensing of nerve gases. Nano Lett 5:649–653. doi:10.1021/nl050054c
Inacio Lunkes G, Stefanello F, Sausen Lunkes D, Maria Morsch V, Schetinger MR, Goncalves JF (2006) Serum cholinesterase activity in diabetes and associated pathologies. Diabetes Res Clin Pract 72:28–32. doi:10.1016/j.diabres.2005.08.009
Hampton MB, Kettle AJ, Winterbourn CC (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017
Regasini LO, Vellosa JC, Silva DH, Furlan M, de Oliveira OM, Khalil NM, Brunetti IL, Young MC, Barreiro EJ, Bolzani VS (2008) Flavonols from Pterogyne nitens and their evaluation as myeloperoxidase inhibitors. Phytochemistry 69:1739-44. doi:10.1016/j.phytochem.2008.01.006
Molina MF, Sanchez-Reus I, Iglesias I, Benedi J (2003) Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull 26:1398–1402
Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB (2002) Flavonol and flavone intakes in US health professionals. J Am Diet Assoc 102:1414–1420
Choi EJ, Chee KM, Lee BH (2003) Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol 482:281–285
Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ (2006) Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav Brain Res 171:251–260. doi:10.1016/j.bbr.2006.03.043
Zalups RK, Ahmad S (2003) Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186:163–188
Santos FW, Oro T, Zeni G, Rocha JB, do Nascimento PC, Nogueira CW (2004) Cadmium induced testicular damage and its response to administration of succimer and diphenyl diselenide in mice. Toxicol Lett 152:255–263. doi:10.1016/j.toxlet.2004.05.009
Goncalves JF, Duarte MM, Fiorenza AM, Spanevello RM, Mazzanti CM, Schmatz R, Bagatini MD, Antes FG, Costa P, Abdalla FH, Dressler VL, Morsch VM, Schetinger MR (2012) Hematological indices and activity of NTPDase and cholinesterase enzymes in rats exposed to cadmium and treated with N-acetylcysteine. Biometals 25:1195–1206. doi:10.1007/s10534-012-9582-2
Abdalla FH, Cardoso AM, Pereira LB, Schmatz R, Goncalves JF, Stefanello N, Fiorenza AM, Gutierres JM, Serres JD, Zanini D, Pimentel VC, Vieira JM, Schetinger MR, Morsch VM, Mazzanti CM (2013) Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol Cell Biochem 381:1–8. doi:10.1007/s11010-013-1659-x
Braganhol E, Tamajusuku AS, Bernardi A, Wink MR, Battastini AM (2007) Ecto-5′-nucleotidase/CD73 inhibition by quercetin in the human U138MG glioma cell line. Biochim Biophys Acta 1770:1352–1359. doi:10.1016/j.bbagen.2007.06.003
Rockenbach L, Bavaresco L, Fernandes Farias P, Cappellari AR, Barrios CH, Bueno Morrone FO, Battastini AM (2013) Alterations in the extracellular catabolism of nucleotides are involved in the antiproliferative effect of quercetin in human bladder cancer T24 cells. Urol Oncol 31:1204–1211. doi:10.1016/j.urolonc.2011.10.009
Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97:77–89
Bergmeyer H (1983) Methods of enzymatic analysis. Verlag Chemie, Deerfiled Beach
Leal DB, Streher CA, Neu TN, Bittencourt FP, Leal CA, da Silva JE, Morsch VM, Schetinger MR (2005) Characterization of NTPDase (NTPDase1; ecto-apyrase; ecto-diphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes. Biochim Biophys Acta 1721:9–15. doi:10.1016/j.bbagen.2004.09.006
Chan K, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-ATPase activity. Anal Biochem 1986;157:375-8
Giusti G, Gakis C (1971) Temperature conversion factors, activation energy, relative substrate specificity and optimum pH of adenosine deaminase from human serum and tissues. Enzyme 12:417–425
Ellman GL, Courtney KD, Andres V, Jr Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fitzgerald BB, Costa LG (1993) Modulation of muscarinic receptors and acetylcholinesterase activity in lymphocytes and in brain areas following repeated organophosphate exposure in rats. Fundam Appl Toxicol 20:210–216
Metcalf J, Gallin WN, Nauseef R (1986) Root, laboratory manual of neutrophil function. Raven Press, New York
Kayyali US, Moore TB, Randall JC, Richardson RJ (1991) Neurotoxic esterase (NTE) assay: optimized conditions based on detergent-induced shifts in the phenol/4-aminoantipyrine chromophore spectrum. J Anal Toxicol 15:86–89
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Murugavel PP, Pari L (2007) Diallyl tetrasulfide modulates the cadmium-induced impairment of membrane bound enzymes in rats. J Basic Clin Physiol Pharmacol 18:37–48
Thome GR, Mazzanti CM, Ahmed M, Correa M, Spanevello RM, Maldonado PA, Luchese C, Cargnelutti D, Morsch VM, Duarte MM, Fiorenza AM, Nogueira CW, De Bona KS, Moretto MB, Da Luz SC, Mazzanti A, Schetinger MR (2009) Activity of ectonucleotidases and adenosine deaminase in rats exposed to cigarette smoke. Inhal Toxicol 21:906–912. doi:10.1080/08958370802632267
Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11:201–212. doi:10.1038/nri2938
Scheuplein F, Schwarz N, Adriouch S, Krebs C, Bannas P, Rissiek B, Seman M, Haag F, Koch-Nolte F (2009) NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol 182:2898–2908. doi:10.4049/jimmunol.0801711
Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C (2006) The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol 13:319–328. doi:10.1128/CVI.13.3.319-328.2006
Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung W, Lin WW (2005) Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 521:9–20. doi:10.1016/j.ejphar.2005.08.005
Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, Song JC, Jeong KS (2003) Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem 243:153–160
Marzena SM, Mateusz K (2012) Review article: flavonoids and their properties to form chelate complexes. Biotechnol Food Sci 76:35–41
Dehghan G, Khoshkam Z (2011) Chelation of toxic Tin(II) by quercetin: a spectroscopic study. Int Conf Life Sci Technol 3:1–3
Rajendran M, Ravichandran R, Devapiriam D (2012) Molecular modeling study of quercetin and their metal complexes. Int J Comput Appl 50:30–34
Jaques J, Rezer J, Ruchel J, Becker L, CS R, Luz S, Gutierres J, JF G, Morch V, Schetinger M, Leal D (2011) Lung and blood lymphocytes NTPDase and acetylcholinesterase activity in cigarette smoke-exposed rats treated with curcumin. Biomed Prevent Nutr 1:109–115
Kaizer RR, Gutierres JM, Schmatz R, Spanevello RM, Morsch VM, Schetinger MR, Rocha JB (2010) In vitro and in vivo interactions of aluminum on NTPDase and AChE activities in lymphocytes of rats. Cell Immunol 265:133–138. doi:10.1016/j.cellimm.2010.08.001
Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T (2006) Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology 50:540–547. doi:10.1016/j.neuropharm.2005.10.013
Mazzanti CM, Spanevello R, Ahmed M, Pereira LB, Goncalves JF, Correa M, Schmatz R, Stefanello N, Leal DB, Mazzanti A, Ramos AT, Martins TB, Danesi CC, Graca DL, Morsch VM, Schetinger MR (2009) Pre-treatment with ebselen and vitamin E modulate acetylcholinesterase activity: interaction with demyelinating agents. Int J Dev Neurosci 27:73–80. doi:10.1016/j.ijdevneu.2008.09.005
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol 42:1563–1571. doi:10.1016/j.fct.2004.05.001
Loizzo MR, Tundis R, Menichini F (2008) Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr Med Chem 15:1209–1228
Manjeet KR, Ghosh B (1999) Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol 21:435–443
Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ (2007) Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr 137:2190–2195
Melzig MF (1996) Inhibition of adenosine deaminase activity of aortic endothelial cells by selected flavonoids. Planta Med 62:20–21. doi:10.1055/s-2006-957788
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC (2007) Adenosine generation catalysed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Coordenação e Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Federal University of Santa Maria, RS, Brazil, and the FINEP research grant "Rede Instituto Brasileiro de Neurociências (IBNet)”, Instituto Nacional de Ciências Tecnológicas (INCT).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdalla, F.H., Cardoso, A.M., Schmatz, R. et al. Protective effect of quercetin in ecto-enzymes, cholinesterases, and myeloperoxidase activities in the lymphocytes of rats exposed to cadmium. Mol Cell Biochem 396, 201–211 (2014). https://doi.org/10.1007/s11010-014-2155-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2155-7