Abstract
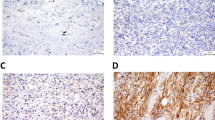
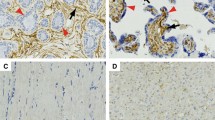
Different forms of sarcoma (solid or ascitic) often pose a critical medical situation for pediatric or adolescent group of patients. To date, predisposed genetic anomalies and related changes in protein expression are thought to be responsible for sarcoma development. However, in spite of genetic abnormality, role of tumor microenvironment is also indispensable for the evolving neoplasm. In our present study, we characterized the deferentially remodeled microenvironment in solid and ascitic tumors by sequential immunohistochemistry and flowcytometric analysis of E-cdaherin, N-cadherin, vimentin, and cytokeratin along with angiogenesis and metastasis. In addition, we considered flowcytometric apoptosis and CD133 positive cancer stem cell analysis. Comparative hemogram was also considered as a part. Our investigation revealed that both types of tumor promoted neovascularization over time with sign of local inflammation. Invasion of neighboring skeletal muscle by solid sarcoma was more frequent than its ascitic counterpart. In contrary, rapid and earlier cadherin switching (E-cadherin to N-cadherin) in ascitic sarcoma made them more aggressive than that of solid sarcoma and helped to early metastasize distant tissue like liver through the hematogenous route. Differential cadherin switching and infidelity of cytokeratin expression in Vimentin positive sarcoma also influenced the behavior of ascitic CD133+ cancer initiating cell pool with respect to CD133+ cells housed in solid sarcoma. Therefore our study concludes that differential cadherin switching program and infidelity of intermediate filaments in part, sharply discriminate the severity and metastatic potentiality of either type of sarcoma accompanying with CD133+ cellular repertoire. Besides, tumor phenotype-based dichotomous cadherin switching program could be exploited as a future drug target to manage decompensated malignant ascitic and solid sarcoma.







Similar content being viewed by others
References
Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R (2010) Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol 21:1106–1111. doi:10.1093/annonc/mdp415
Berx G, Van Roy F (2001) The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 3:289–293. doi:10.1186/bcr309
Cairns R, Papandreou I, Denko N (2006) Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res 4:61–70. doi:10.1158/1541-7786.MCR-06-0002
Derycke LD, Bracke ME (2004) N-cadherin in the spotlight of cell–cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol 48:463–476. doi:10.1387/ijdb.041793ld
Gatenby RA, Gawlinski ET (2003) The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical model. Cancer Res 63:3847–3854
Gillies RJ, Gatenby RA (2007) Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr 39:251–257. doi:10.1007/s10863-007-9085-y
Griffiths JR (1991) Are cancer cells acidic? Br J Cancer 64:425–427. doi:10.1038/bjc.1991.326
Nicole NP, Dietmar WS (2001) The microenvironment in cancer tumor. In: Dietmar WS (ed) Tumor microenvironment. Wiley, Gainesville, pp 1–6
Stasinopoulos I, Peneta M, Chen Z, Kakkad S, Glunde K, Bhujwalla ZM (2011) Exploiting the tumor microenvironment for theranostic imaging. NMR Biomed 24:636–647. doi:10.1002/nbm.1664
Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454. doi:10.1093/jnci/djm135
Castells M, Thibault B, Delord JP, Couderc B (2012) Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci 13:9545–9571. doi:10.3390/ijms13089545
Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL (2006) Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res 66:2725–2731. doi:10.1158/0008-5472.CAN-05-3719
Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K (2009) Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci USA 106:3372–3377. doi:10.1073/pnas.0813306106
Bilici A, Uygun K, Altun E, Karci E, Gurbuz Y, Semih Dogan A (2009) Primary peritoneal spindle cell sarcoma presented with massive ascites: a case report. J BUON 14:721–723
Oei TN, Jagannathan JP, Ramaiya N, Ros PR (2010) Peritoneal sarcomatosis versus peritoneal carcinomatosis: imaging findings at MDCT. Am J Roentgenol 195:W229–W235. doi:10.2214/AJR.09.3907
Yokoi M, Hosokawa K, Funaki H, Yoshitani S, Kinami S, Omote K, Ueda N, Nakano Y, Kosaka T, Minato H (2009) A case of retroperitoneal dedifferentiated liposarcoma successfully treated with IFM and CDDP. Gan To Kagaku Ryoho 36:2114–2116
Colizza S, Di Maurizio P, Bracci F, Bigotti A, Crisci E (1981) Diffuse peritoneal liposarcoma-report of one case. J Surg Oncol 18:147–151. doi:10.1002/jso.2930180207
Leal R, Lewin M, Ahmad I, Korula J (1994) Peritoneal Kaposi’s sarcoma: a cause of ascites in acquired immunodeficiency syndrome. Dig Dis Sci 39:206–208. doi:10.1007/BF02090084
Mimeault M, Batra SK (2007) Interplay of distinct growth factors during epithelial–mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol 18:1605–1619. doi:10.1093/annonc/mdm070
Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR (2000) N-cadherin extracellular repeat four mediates epithelial to mesenchymal transition and increased motility. J Cell Biol 151:1193–1206. doi:10.1083/jcb.151.6.1193
Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G (1998) A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392:190–193. doi:10.1038/32433
Hazan RB, Kang L, Whooley BP, Borgen PI (1997) N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun 4:399–411
Kamei J, Toyofuku T, Hori M (2003) Negative regulation of p21 by β catenin/TCF signaling: a novel mechanism by which cell adhesion molecules regulate cell proliferation. Biochem Biophys Res Commun 312:380–387. doi:10.1016/j.bbrc.2003.10.129
Schoumacher M, Goldman RD, Louvard D, Vignjevic DM (2010) Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 189:541–556. doi:10.1083/jcb.200909113
Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G (2011) Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J 25:2022–2030. doi:10.1096/fj.10-179036
Jiang X, Gwye Y, Russell D, Cao C, Douglas D, Hung L, Kovar H, Triche TJ, Lawlor ER (2010) CD133 expression in chemo-resistant Ewing sarcoma cells. BMC Cancer 10:116. doi:10.1186/1471-2407-10-116
Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I (2009) Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res 69:1776–1781. doi:10.1158/0008-5472.CAN-08-2242
Terry J, Nielsen T (2010) Expression of CD133 in synovial sarcoma. Appl Immunohistochem Mol Morphol 18:159–165. doi:10.1097/PAI.0b013e3181b77451
Stratford EW, Castro R, Wennerstrom A, Holm R, Munthe E, Lauvrak S, Bjerkehagen B, Myklebost O (2011) Liposarcoma cells with aldefluor and CD133 activity have a cancer stem cell potential. Clin Sarcoma Res 1:1–11. doi:10.1186/2045-3329-1-8
Zaslavsky A, Chen C, Grillo J, Baek KH, Holmgren L, Yoon SS, Folkman J, Ryeom S (2010) Regional control of tumor growth. Mol Cancer Res 8:1198–1206. doi:10.1158/1541-7786.MCR-10-0047
Chaklader M, Das P, Pereira JA, Law A, Chattopadhyay S, Chatterjee R, Mondal A, Law S (2012) 17-AAG mediated targeting of Hsp90 limits TERT activity in peritoneal sarcoma related malignant ascites by downregulating cyclin D1 during cell cycle entry. Exp Oncol 34:90–96
Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR (2008) Cadherin switching. J Cell Sci 121:727–735. doi:10.1242/jcs.000455
Hong S, Troyanovsky RB, Troyanovsky SM (2011) Cadherin exits the junction by switching its adhesive bond. J Cell Biol 192(6):1073–1083. doi:10.1083/jcb.201006113
Tomita K, van Bokhoven A, van Leenders GJLH, Ruijter ETG, Jansen C, Bussemakers MJG, Schalken JA (2000) Cadherin switching in human prostate cancer progression. Cancer Res 60:3650–3654
Tian W, Wang G, Yang J, Pan Y, Ma Y (2013) Prognostic role of E-cadherin and vimentin expression in various subtypes of soft tissue leiomyosarcomas. Med Oncol 30:401. doi:10.1007/s12032-012-0401-y
Püsküllüoglu M, Lukasiewicz E, Miekus K, Jarocha D, Majka M (2010) Differential expression of Snail1 transcription factor and Snail1-related genes in alveolar and embryonal rhabdomyosarcoma subtypes. Folia Histochem Cytobiol 48:671–677. doi:10.2478/v10042-010-0046-7
Subramaniam MM, Navarro S, Llombart-Bosch A (2011) Immunohistochemical study of correlation between histologic subtype and expression of epithelial-mesenchymal transition-related proteins in synovial sarcomas. Arch Pathol Lab Med 135:1001–1009. doi:10.5858/2010-0071-OAR1
Laskin WB, Miettinen M (2002) Epithelial-type and neural-type cadherin expression in malignant noncarcinomatous neoplasms with epithelioid features that involve the soft tissues. Arch Pathol Lab Med 126:425–431
Saito T, Oda Y, Sugimachi K, Kawaguchi K, Tamiya S, Tanaka K, Matsuda S, Sakamoto A, Iwamoto Y, Tsuneyoshi M (2001) E-cadherin gene mutations frequently occur in synovial sarcoma as a determinant of histological features. Am J Pathol 159:2117–2124
Ureshino H, Murakami Y, Watari K, Izumi H, Kawahara A, Kage M, Arao T, Nishio K, Yanagihara K, Kinoshita H, Kuwano M, Ono M (2012) N-myc downstream regulated gene 1 (NDRG1) promotes metastasis of human scirrhous gastric cancer cells through epithelial mesenchymal transition. PLoS One 7:e41312. doi:10.1371/journal.pone.0041312
Acknowledgments
All authors are thankful to the director of the Calcutta School of Tropical Medicine.
Conflict of interest
All authors unanimously declared no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaklader, M., Pan, A., Law, A. et al. Differential remodeling of cadherins and intermediate cytoskeletal filaments influence microenvironment of solid and ascitic sarcoma. Mol Cell Biochem 382, 293–306 (2013). https://doi.org/10.1007/s11010-013-1750-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1750-3