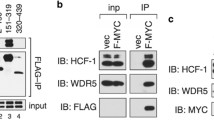
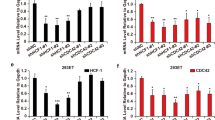
Abstract
Host Cell Factor (HCF-1) is a conserved, essential protein initially identified as a co-regulator for the Herpes Simplex Virus transactivator VP16. HCF-1 is variously involved in regulating transcription, splicing, cell proliferation and cytokinesis; however, its mechanisms of action remain unknown. HCF-1 function is manifested through an increasing assortment of cellular factors that target different regions of the protein. Several HCF-1 partners target the amino-terminal kelch domain of HCF-1 (residues 1–380) via a consensus HCF-binding motif (HBM) comprising the tetrapeptide (D/E)HXY. Searches of sequence databases indicated that this motif is present in E2F1 and E2F4, two members of the E2F family of cell cycle regulators. We show here that E2F4 specifically and directly interacts with HCF-1. Mutational analysis showed E2F4 independently targets the kelch domain and the basic domain (residues 450–902) of HCF-1, both of which are required for normal cell-cycle progression via separate determinants. The HBM-containing domain of E2F4 was necessary for interaction with the kelch domain of HCF-1 but not for interaction with the basic domain. Mutations in the HCF-1 kelch domain known to block cell growth abrogated E2F4 binding to the kelch domain in the absence but not in the presence of the juxtaposed basic region. Functionally, HCF-1 co-activated E2F4/DP-1 in transient transfection assays, while E2F4 blocked HCF-1-dependent rescue of a cell line that harbors a temperature sensitive mutant of HCF-1 that causes growth arrest. Our findings show that HCF-1 and E2F4 interact via multiple determinants and suggest a linkage between E2F4 and HCF-1 cell growth pathways.
Similar content being viewed by others
Abbreviations
- HCF-1:
-
Host Cell Factor 1
- HBM:
-
HCF-1 binding motif
- AAD:
-
acidic activation domain
- HA:
-
haemagglutinin
- Rb:
-
retinoblastoma
- wt:
-
wild-type
References
Wysocka J, Herr W: The herpes simplex virus VP16-induced complex: The makings of a regulatory switch. Trends Biochem Sci 28: 294–304, 2003
Luciano RL, Wilson AC: HCF-1 functions as a coactivator for the zinc finger protein Krox20. J Biol Chem 278: 51116–51124, 2003
Goto H, Motomura S, Wilson AC, Freiman RN, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T: A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes Dev 11: 726–737, 1997
Reilly PT, Herr W: Spontaneous reversion of tsBN67 cell proliferation and cytokinesis defects in the absence of HCF-1 function. Exp Cell Res 277: 119–130, 2002
Julien E, Herr W: Proteolytic processing is necessary to separate and ensure proper cell growth and cytokinesis functions of HCF-1. EMBO J 22: 2360–2369, 2003
Wysocka J, Reilly PT, Herr W: Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Mol Cell Biol 21: 3820–3829, 2001
Julien, E, Herr W: A switch in mitotic histone H4 lysine 20 methylation status is linked to M phase defects upon loss of HCF-1. Mol Cell 14: 713–725, 2004
Wilson AC, LaMarco K, Peterson MG, Herr W: The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell 74: 115–125, 1993
Vogel JL, Kristie TM: Autocatalytic proteolysis of the transcription factor-coactivator C1 (HCF): a potential role for proteolytic regulation of coactivator function. Proc Natl Acad Sci USA 97: 9425–9430, 2000
Wilson AC, Boutros M, Johnson KM, Herr W: HCF-1 amino- and carboxy-terminal subunit association through two separate sets of interaction modules: involvement of fibronectin type 3 repeats. Mol Cell Biol 20: 6721–6730, 2000
Adams J, Kelso R, Cooley L: The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol 10: 17–24, 2000
Lu R, Yang P, Padmakunar S, Misra V: The herpesvirus transactivator VP16 mimics a human basic domain leucine zipper protein, luman, in its interaction with HCF. J Virol 72: 6291–6297, 1998
Lu R, Misra V: Zhangfei: A second cellular protein interacts with herpes simplex virus accessory factor HCF in a manner similar to Luman and VP16. Nucleic Acids Res 28: 2446–2454, 2000
Lin J, Puigserver P, Donovan J, Tarr, P, Spiegelman BM: Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277: 1645–1648, 2002
Mahajan SS, Little MM, Vazquez R, Wilson AC: Interaction of HCF-1 with a cellular nuclear export factor. J Biol Chem 277: 44292–44299, 2002
Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W: Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev 17: 896–911, 2003
Gunther M, Laithier M, Brison O: A set of proteins interacting with transcription factor Sp1 identified in a two-hybrid screening. Mol Cell Biochem 210: 131–142, 2000
Vogel JL, Kristie TM: The novel coactivator C1 (HCF) coordinates multiprotein enhancer formation and mediates transcription activation by GABP. EMBO J 19: 683–690, 2000
Piluso D, Bilan P, Capone JP: Host cell factor-1 interacts with and antagonizes transactivation by the cell cycle regulatory factor Miz-1. J Biol Chem 277: 46799–46808, 2002
Ajuh PM, Browne GJ, Hawkes NA, Cohen PT, Roberts SG, Lamond AI: Association of a protein phosphatase 1 activity with the human factor C1 (HCF) complex. Nucleic Acids Res 28: 678–686, 2000
Scarr RB, Sharp PA: PDCD2 is a negative regulator of HCF-1 (C1). Oncogene 21: 5245–5254, 2003
Wilson AC, Freiman RN, Goto H, Nishimoto T, Herr W: VP16 targets an amino-terminal domain of HCF involved in cell cycle progression. Mol Cell Biol 17: 6139–6146, 1997
Mahajan SS, Wilson AC: Mutations in host cell factor 1 separate its role in cell proliferation from recruitment of VP16 and LZIP. Mol Cell Biol 20: 919–928, 2000
Freiman RN, Herr W: Viral mimicry: Common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev 11: 3122–3127, 1997
Dyson N: The regulation of E2F by pRB-family proteins. Genes Dev 12: 2245–2262, 1998
Trimarchi JM, Lees JA: Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 31: 11–20, 2002
deBruin A, Maiti B, Jakoi L, Timmers C, Buerki R, Leone G: Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem 278: 42041–42049, 2003
Beijersbergen RL, Kerkhoven RM, Zhu L, Carlee L, Voorhoeven PM, Bernards R: E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev 8: 2680–2690, 1994
Ginsberg D, Vairo G, Chittenden T, Xiao Z-X, Xu G, Wydner KL, DeCaprio JA, Lawrence JB, Livingston DM: E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev 8: 2665–2679, 1994
Vairo G, Livingston DM, Ginsberg D: Functional interaction between E2F-4 and p130:evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 9: 869–881, 1995
Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K: E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev 15: 267–285, 2001
Li J-M, Hu PP, Shen X, Yu Y, Wang X-F: E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor beta through E2F binding sites. Proc Natl Acad Sci USA 94: 4948–4953, 1997
Takahashi Y, Rayman JB, Dynlacht BD: Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev 14: 804–16, 2000
Wang D, Russell JL, Johnson DG: E2F4 and E2F1 have similar proliferative properties but different apoptotic and oncogenic properties in vivo. Mol Cell Biol 20: 3417–3424, 2000
Knez J, Bilan PT, Capone JP: A single amino acid substitution in herpes simplex virus type 1 VP16 inhibits binding to the virion host shutoff protein and is incompatible with virus growth. J Virol 77: 2892–2902, 2003
Luciano RL, Wilson AC: An activation domain in the C-terminal subunit of HCF-1 is important for transactivation by VP16 and LZIP. Proc Natl Acad Sci USA 99: 13403–13408, 2002
Ajuh P, Chusainow J, Ryder U, Lamond AI: A novel function for human factor C1 (HCF-1), a host protein required for herpes simplex virus infection, in pre-mRNA splicing. EMBO J 21: 6590–6602, 2002
Weinmann AS, Yan PS, Oberley MJ, Huang TH, Farnham PJ: Isolating human transcription factor targets by coupling chromatin immunoprecipitation and CpG island microarray analysis. Genes Dev 16: 235–244, 2002
Rayman JB, Takahashi Y, Indjeian VB, Dannenberg JH, Catchpole S, Watson RJ, te Riele H, Dynlacht BD: E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev 16: 933–947, 2002
Lang SE, McMahon SB, Cole MD, Hearing P: E2F transcriptional activation requires TRRAP and GCN5 cofactors. J Biol Chem 276: 32627–32634, 2001
Reilly PT, Wysocka J, Herr W: Inactivation of the retinoblastoma protein family can bypass the HCF-1 defect in tsBN67 cell proliferation and cytokinesis. Mol Cell Biol 22: 6767–78, 2002
Wanzel M, Herold S, Eilers M: Transcriptional repression by Myc. Trends Cell Biol 13: 146–150, 2003
Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Moroy T, Bartek J, Massague J, Hanel F, Eilers M: Repression of p15INK4b expression by Myc through association with Miz-1. Nature Cell Biol 3: 392–399, 2001
Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J: TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nature Cell Biol 3: 400–408, 2001
Chen C-R, Kang Y, Siegel PM, Massague J: E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 110: 19–32, 2002
Stevens C, LaThangue NB: E2F and cell cycle control: a double-edged sword. Arch Biochem Biophys 412: 157–69, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knez, J., Piluso, D., Bilan, P. et al. Host Cell Factor-1 and E2F4 Interact Via Multiple Determinants in Each Protein. Mol Cell Biochem 288, 79–90 (2006). https://doi.org/10.1007/s11010-006-9122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9122-x