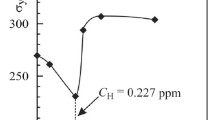
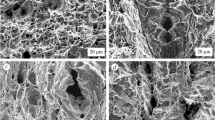
We establish the specific features of the process of deformation of low-alloy pipe steel in hydrogencontaining media depending on the bulk concentration of hydrogen in the metal. We determine a characteristic value of concentration for which the mechanism of the influence hydrogen of on the deformation of steel changes: Below this value, the material undergoes plasticization and, above this value, it suffers embrittlement. The value C ∗H can be regarded as an important engineering parameter for the evaluation of strength and the analysis of fracture processes in materials and structural elements in hydrogen-containing media and for the development of new technologies of hydrogen treatment of structural materials with an aim to optimize their operating characteristics.







Similar content being viewed by others
References
B. Somerday, P. Sofronis, and R. Jones (editors), Effects of Hydrogen on Materials, Pro. of the 2008 Internat. Hydrogen Conf. (September 7–10, 2008 ), ASM International, Materials Park (2009).
J. W. Hanneken, “Hydrogen in metals and other materials: a comprehensive reference to books, bibliographies, workshops and conferences,” Int. J. Hydrogen Energy, 24, No. 10, 1005–1026 (1999).
W. Godoi, N. K. Kuromoto, A. S. Guimarães, et al., “Effect of the hydrogen outgassing time on the hardness of austenitic stainless steels welds,” Mater. Sci. Eng. A, 354, No. 1–2, 251–256 (2003).
V. G. Gavriljuk, V. N. Shivanjuk, and J. Foct, “Diagnostic experimental results on the hydrogen embrittlement of austenitic steels,” Acta Mater., 51, No. 5, 1293–1305 (2003).
GOST 1497–84. Methods for Tensile Testing [in Russian], Izd. Standartov, Moscow (1985).
G. Z. Meng, C. Zhang, and Y. F. Cheng, “Effects of corrosion product deposit on the subsequent cathodic and anodic reactions of X-70 steel in near-neutral pH solution,” Corr. Sci., 50, No. 11, 3116–3122 (2008).
L. Niu and Y. F. Cheng, “Corrosion behavior of X-70 pipe steel in near-neutral pH solution,” Appl. Surface Sci., 253, No. 21, 8626–8631 (2007).
B. T. Lu, J. L. Luo, P. R. Norton, et al., “Effects of dissolved hydrogen and elastic and plastic deformation on active dissolution of pipeline steel in anaerobic groundwater of near-neutral pH,” Acta Mater., 57, No. 1, 41–49 (2009).
Y. F. Cheng and L. Niu, “Mechanism for hydrogen evolution reaction on pipeline steel in near-neutral pH solution,” Electrochem. Comm., 9, No. 4, 558–562 (2007).
Y. F. Cheng, “Fundamentals of hydrogen evolution reaction and its implications on near-neutral pH stress corrosion cracking of pipelines,” Electrochem. Acta, 52, No. 7, 2661–2667 (2007).
S. A. Shipilov and I. L. May, “Structural integrity of aging buried pipelines having cathodic protection,” Eng. Fail. Anal., 13, No. 7, 1159–1176 (2006).
S. Dey, A. K. Mandhyan, S. K. Sondhi, et al., “Hydrogen entry into pipeline steel under freely corroding conditions in two corroding media,” Corr. Sci., 48, No. 9, 2676–2688 (2006).
J. Capelle, J. Gilgert, I. Dmytrakh, et al., “Sensitivity of pipelines with steel API X52 to hydrogen embrittlement,” Int. J. Hydrogen Energy, 33, No. 24, 7630–7641 (2008).
M. Yan and Y. Weng, “Study on hydrogen absorption of pipeline steel under cathodic charging,” Corr. Sci., 48, No. 2, 432–444 (2006).
VoltaLab 40 (PGZ301 & VoltaMaster 4). Dynamic Electrochemical Laboratory. Instruction, Radiometer Analytical (2009).
O. E. Andreikiv and S. T. Shtayura, Experimental Mechanics of Materials: Tutorial, Part 1: Force Factors. Isotropic Materials Laboratory Practice) [in Ukrainian], Franko Lviv National University, Lviv (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 50, No. 2, pp. 16–23, March–April, 2014.
Rights and permissions
About this article
Cite this article
Dmytrakh, І.M., Leshchak, R.L., Syrotyuk, A.M. et al. Influence of the Bulk Concentration of Hydrogen in the Metal on the Specific Features of Deformation of Low-Alloy Pipe Steel. Mater Sci 50, 170–178 (2014). https://doi.org/10.1007/s11003-014-9706-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-014-9706-7