Abstract
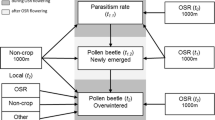
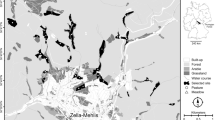
Linking spatial pattern and process is a difficult task in landscape ecology because spatial patterns of populations result from complex factors such as individual traits, the spatio-temporal variation of the habitat, and the relationships between the target species and other species. Mechanistic models provide tools to bridge this gap but they are seldom used to study the influence of landscape patterns on biological processes. In this paper, we develop a methodological approach based on sensitivity and multivariate analyses to investigate the relationship between the biological parameters of species and landscape characteristics. As a case study, we used a tritrophic system that includes a host plant (oilseed rape, Brassica napus L.), a pest of the host plant (the pollen beetle, Meligethes aeneus F.), and the main parasitoid of the pest (Tersilochus heterocerus). This tritrophic system was recently represented by a model (Mosaic-Pest) that is spatially explicit at the landscape scale and that includes 32 biological parameters. In the current study, model simulations were compared with observed data from 35 landscapes differing in configuration. Sensitivity analysis using the Morris method identified those biological parameters that were highly sensitive to landscape configuration. Then, multivariate analyses revealed how a parameter’s influence on model output could be affected by landscape composition. Comparison of simulated and observed data helped us decrease the uncertainty surrounding the estimated values of the literature-derived parameters describing beetle dispersal and stage transition of the parasitoid at emergence. The advantages of using multivariate sensitivity analyses to disentangle the links between patterns and processes in landscape-scale spatially explicit models are discussed.






Similar content being viewed by others
References
Beaudouin R, Monod G, Ginot V (2008) Selecting parameters for calibration via sensitivity analysis: an individual-based model of mosquito fish population dynamics. Ecol Model 218:29–48
Bianchi FJJA, Schellhorn NA (2009) Foraging behaviour of predators in heterogeneous landscapes: the role of perceptual ability and diet breadth. Oikos 118:1363–1372
Cariboni J, Gatelli D, Liska R, Saltelli A (2007) The role of sensitivity analysis in ecological modelling. Ecol Model 203:167–182
Charnell MA (2008) An individual-based model of a tritrophic ecology. Ecol Model 218:195–206
Cook S, Murray D, Williams I (2004) Do pollen beetles need pollen? The effect of pollen on oviposition, survival, and development of a flower-feeding herbivore. Ecol Entomol 29:164–173
Cook SM, Khan ZR, Pickett JA (2007) The use of push-pull strategies in integrated pest management. Annu Rev Entomol 52:375–400
Csilléry K, Blum MGB, Gaggiotti OE, François O (2010) Approximate Bayesian computation (ABC) in practice. Trends Ecol Evol 25:410–418
Dray S, Chessel D, Thioulouse J (2003) Co-inertia analysis and the linking of ecological data tables. Ecology 84:3078–3089
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Frair JL, Merrill EH, Visscher DR, Fortin D, Beyer HL, Morales JM (2005) Scales of movement by elk (Cervus elaphus) in response to heterogeneity in forage resources and predation risk. Landscape Ecol 20:273–287
Grimm V, Revilla E, Berger U, Jeltsch F, Mooij WM, Railsback SF, Thulke HH, Weiner J, Wiegand T, DeAngelis DL (2005) Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science 310:987–991
Hartig F, Calabrese JM, Reineking B, Wiegand T, Huth A (2011) Statistical inference for stochastic simulation models—theory and application. Ecol Lett 14:816–827
Herrström G (1964) Untersuchungen über Parasiten von Ölfruchtschädlingen in Sweden. Meddn StVäxtskAnst 12:433–448
Jourdheuil P (1960) Influence de quelques facteurs écologiques sur les fluctuations de population d’une biocénose parasitaire. INRA, Paris
Kramer-Schadt S, Revilla E, Wiegand T, Breitenmoser U (2004) Fragmented landscapes, road mortality and patch connectivity: modelling influences on the dispersal of Eurasian lynx. J Appl Ecol 41:711–723
Kramer-Schadt S, Revilla E, Wiegand T, Grimm V (2007) Patterns for parameters in simulation models. Ecol Model 204:553–556
Luxmoore RJ, King AW, Tharp ML (1991) Approaches to scaling up physiologically based soil–plant models in space and time. Tree Physiol 9:281–292
Martinez I, Wiegand T, Batllori E, Gutierrez E (2011) Disentangling the formation of contrasting tree-line physiognomies combining model selection and Bayesian parameterization for simulation models. Am Nat 177:E136–E152
McIntire EJB, Fajardo A (2009) Beyond description: the active and effective way to infer processes from spatial patterns. Ecology 90:46–56
Mills NJ, Getz WM (1996) Modelling the biological control of insect pests: a review of host–parasitoid models. Ecol Model 92:121–143
Monod H, Naud C, Makowski D (2006) Uncertainty and sensitivity analysis for crop models. In: Wallach D, Makowski D, Jones J (eds) Working with dynamic crop models. Elsevier, Amsterdam
Morris MD (1991) Factorial sampling plans for preliminary computational experiments. Technometrics 33:161–174
Nilsson C (1988a) The pollen beetle (Meligethes aeneus F.) in winter and spring rape at Alnarp 1976–1978. I. Migration and sex ratio. Växtskyddsnotiser 56:6
Nilsson C (1988b) The pollen beetle (Meligethes aeneus F.) in winter and spring rape at Alnarp 1976–1978. II. Oviposition. Växtskyddsnotiser 52: 139–144
Nilsson C (2010) Impact of soil tillage on parasitoids of oilseed rape pests. In: Williams I (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, Dordrecht, pp 45–76
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160
Ricci B, Franck P, Toubon JF, Bouvier JC, Sauphanor B, Lavigne C (2009) The influence of landscape on insect pest dynamics: a case study in southeastern France. Landscape Ecol 24:337–349
Rusch A, Valantin-Morison M, Sarthou J-P, Roger-Estrade J (2011) Multi-scale effects of landscape complexity and crop management on pollen beetle parasitism rate. Landscape Ecol 26:473–486
Saltelli A, Tarantola S, Campolongo F (2000) Sensitivity analysis as an ingredient of modeling. Stat Sci 15:377–395
Stonedahl F, Wilensky U (2010) Evolutionary robustness checking in the artificial Anasazi model. In: Proceedings of the AAAI fall symposium on complex adaptive systems: resilience, robustness, and evolvability. Arlington, VA, pp 120–129
Taimr L, Sedivy J, Bergmannova E, Hanker I (1967) Further experience obtained in studies on dispersal flights of Meligethes aeneus F., marked with P32 (Coleoptera). Acta Entomol Bohemoslov 64:325–332
ter Braak CJF, Schaffers AP (2004) Co-correspondence analysis: a new ordination method to relate two community compositions. Ecology 85:834–846
Thiele JC, Grimm V (2010) NetLogo meets R: linking agent-based models with a toolbox for their analysis. Environ Model Softw 25:972–974
Tscharntke T, Brandl R (2004) Plant–insect interactions in fragmented landscapes. Annu Rev Entomol 49:405–430
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol Lett 8:857–874
Viaud V, Monod H, Lavigne C, Angevin F, Adamczyk K (2008) Spatial sensitivity of maize gene-flow to landscape pattern: a simulation approach. Landscape Ecol 23:1067–1079
Vinatier F, Gosme M, Valantin-Morison M (2012a) A tool for testing integrated pest management strategies on a tritrophic system involving pollen beetle, its parasitoid and oilseed rape at the landscape scale. Landscape Ecol 27:1421–1433
Vinatier F, Lescourret F, Duyck P-F, Tixier P (2012b) From IBM to IPM: using individual-based models to design the spatial arrangement of traps and crops in integrated pest management strategies. Agric Ecosyst Environ 146:52–59
Wiegand T, Moloney KA, Naves J, Knauer F (1999) Finding the missing link between landscape structure and population dynamics: a spatially explicit perspective. Am Nat 154:605–627
Wiegand T, Knauer F, Kaczensky P, Naves J (2004) Expansion of brown bears (Ursus arctos) into the eastern Alps: a spatially explicit population model. Biodivers Conserv 13:79–114
Wiegand T, Revilla E, Moloney KA (2005) Effects of habitat loss and fragmentation on population dynamics. Conserv Biol 19:108–121
Wilensky U (1999) Netlogo. Center for Connected Learning and Computer-Based Modeling, Evanston
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vinatier, F., Gosme, M. & Valantin-Morison, M. Explaining host–parasitoid interactions at the landscape scale: a new approach for calibration and sensitivity analysis of complex spatio-temporal models. Landscape Ecol 28, 217–231 (2013). https://doi.org/10.1007/s10980-012-9822-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-012-9822-4