Abstract
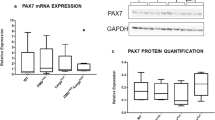
Skeletal muscle myofibers constantly undergo degeneration and regeneration. Histopathological features of 6 skeletal muscles (cranial tibial [CT], gastrocnemius, quadriceps femoris, triceps brachii [TB], lumbar longissimus muscles, and costal part of the diaphragm [CPD]) were compared using C57BL/10ScSn-Dmd mdx (mdx) mice, a model for muscular dystrophy versus control, C57BL/10 mice. Body weight and skeletal muscle mass were lower in mdx mice than the control at 4 weeks of age; these results were similar at 6–30 weeks. Additionally, muscular lesions were observed in all examined skeletal muscles in mdx mice after 4 weeks, but none were noted in the controls. Immunohistochemical staining revealed numerous paired box 7-positive satellite cells surrounding the embryonic myosin heavy chain-positive regenerating myofibers, while the number of the former and staining intensity of the latter decreased as myofiber regeneration progressed. Persistent muscular lesions were observed in skeletal muscles of mdx mice between 4 and 14 weeks of age, and normal myofibers decreased with age. Number of muscular lesions was lowest in CPD at all ages examined, while the ratio of normal myofibers was lowest in TB at 6 weeks. In CT, TB, and CPD, Iba1-positive macrophages, the main inflammatory cells in skeletal muscle lesions, showed a significant positive correlation with the appearance of regenerating myofibers. Additionally, B220-positive B-cells showed positive and negative correlation with regenerating and regenerated myofibers, respectively. Our data suggest that degenerative and regenerative features of myofibers differ among skeletal muscles and that inflammatory cells are strongly associated with regenerative features of myofibers in mdx mice.






Similar content being viewed by others
Abbreviations
- CT:
-
Cranial tibial muscle
- CPD:
-
Costal part of the diaphragm
- GA:
-
Gastrocnemius muscle
- HE:
-
Hematoxylin-eosin
- LL:
-
Lumbar longissimus muscle
- MRF:
-
Myogenic regulatory factor
- MT:
-
Masson’s trichrome stain
- QR:
-
Quadriceps femoris muscle
- TB:
-
Triceps brachii muscle
References
Almekinders LC, Gilbert JA (1986) Healing of experimental muscle strains and the effects of nonsteroidal anti-inflammatory medication. Am J Sports Med 14:303–308
Belcastro AN, Arthur GD, Albisser TA, Raj DA (1996) Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J Appl Physiol (1985) 80(4):1331–1335
Boland B, Himpens B, Denef JF, Gillis JM (1995) Site-dependent pathological differences in smooth muscles and skeletal muscles of the adult mdx mouse. Muscle Nerve 18(6):649–657
Boldrin L, Zammit PS, Morgan JE (2015) Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res 14(1):20–29. doi:10.1016/j.scr.2014.10.007
Brickson S, Ji LL, Schell K, St Pierre Schneider R, Best TM (2003) M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J Appl Physiol (1985) 95(3):969–976
Brussee V, Tardif F, Tremblay JP (1997) Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord 7(8):487–492
Bulfield G, Siller WG, Wight PA, Moore KJ (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81(4):1189–1192
Carpenter JL, Hoffman EP, Romanul FC, Kunkel LM, Rosales RK, Ma NS, Dasbach JJ, Rae JF, Moore FM, McAfee MB, Pearce LK (1989) Feline muscular dystrophy with dystrophin deficiency. Am J Pathol 135(5):909–919
Chargé SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84(1):209–238
Cheung EV, Tidball JG (2003) Administration of the non-steroidal anti-inflammatory drug ibuprofen increases macrophage concentrations but reduces necrosis during modified muscle use. Inflamm Res 52(4):170–176
Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, Scott MO, Fischbeck KH, Kornegay JN, Avery RJ, Williams JR, Schmickel RD, Sylvester JE (1988) The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature 334(6178):154–156
Coulton GR, Morgan JE, Partridge TA, Sloper JC (1988) The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 14(1):53–70
Darr KC, Schultz E (1987) Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol (1985) 63(5):1816–1821
De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, Burdi R, Mangieri D, Rolland JF, Camerino C, Zallone A, Confalonieri P, Andreetta F, Arnoldi E, Courdier-Fruh I, Magyar JP, Frigeri A, Pisoni M, Svelto M, Conte Camerino D (2005) A multidisciplinary evaluation of the effectiveness of cyclosporine a in dystrophic mdx mice. Am J Pathol 166(2):477–489
Emery AE (1989) Clinical and molecular studies in Duchenne muscular dystrophy. Prog Clin Biol 306:15–28
Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D’Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R, Torrente Y (2007) T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 213(2):229–238
Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG (1993) Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol 265(1 Pt 2):R166–R172
Gehrig SM, Koopman R, Naim T, Tjoakarfa C, Lynch GS (2010) Making fast-twitch dystrophic muscles bigger protects them from contraction injury and attenuates the dystrophic pathology. Am J Pathol 176(1):29–33. doi:10.2353/ajpath.2010.090760
Granchelli JA, Pollina C, Hudecki MS (2000) Pre-clinical screening of drugs using the mdx mouse. Neuromuscul Disord 10(4–5):235–239
Grounds MD (1987) Phagocytosis of necrotic muscle in muscle isografts is influenced by the strain, age, and sex of host mice. J Pathol 153(1):71–82
Grounds MD, Torrisi J (2004) Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J 18(6):676–682
Heydemann A, Swaggart KA, Kim GH, Holley-Cuthrell J, Hadhazy M, McNally EM (2012) The superhealing MRL background improves muscular dystrophy. Skelet Muscle 2(1):26. doi:10.1186/2044-5040-2-26
Hindi SM, Shin J, Ogura Y, Li H, Kumar A (2013) Matrix metalloproteinase-9 inhibition improves proliferation and engraftment of myogenic cells in dystrophic muscle of mdx mice. PLoS ONE 8(8):e72121. doi:10.1371/journal.pone.0072121
Hodgetts S, Radley H, Davies M, Grounds MD (2006) Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord 16(9–10):591–602
Le Grand F, Rudnicki M (2007) Satellite and stem cells in muscle growth and repair. Development 134(22):3953–3957
Lescaudron L, Peltékian E, Fontaine-Pérus J, Paulin D, Zampieri M, Garcia L, Parrish E (1999) Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 9(2):72–80
Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A (2009) Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet 18(14):2584–2598. doi:10.1093/hmg/ddp191
Lockhart NC, Brooks SV (2008) Neutrophil accumulation following passive stretches contributes to adaptations that reduce contraction-induced skeletal muscle injury in mice. J Appl Physiol (1985) 104(4):1109–1115. doi:10.1152/japplphysiol.00850.2007
Louboutin JP, Fichter-Gagnepain V, Thaon E, Fardeau M (1993) Morphometric analysis of mdx diaphragm muscle fibers. Comparison with hindlimb muscles. Neuromuscul Disord 3(5–6):463–469
McGeachie JK, Grounds MD, Partridge TA, Morgan JE (1993) Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci 119(2):169–179
McLoughlin TJ, Tsivitse SK, Edwards JA, Aiken BA, Pizza FX (2003) Deferoxamine reduces and nitric oxide synthase inhibition increases neutrophil-mediated myotube injury. Cell Tissue Res 313(3):313–319
Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF (1999) Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22(6):724–732
Mojumdar K, Liang F, Giordano C, Lemaire C, Danialou G, Okazaki T, Bourdon J, Rafei M, Galipeau J, Divangahi M, Petrof BJ (2014) Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR2. EMBO Mol Med 6(11):1476–1492
Morrison J, Lu QL, Pastoret C, Partridge T, Bou-Gharios G (2000) T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest 80(6):881–891
Nguyen HX, Tidball JG (2003a) Expression of a muscle-specific, nitric oxide synthase transgene prevents muscle membrane injury and reduces muscle inflammation during modified muscle use in mice. J Physiol 550(Pt 2):347–356
Nguyen HX, Tidball JG (2003b) Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J Physiol 547(Pt 1):125–132
Orimo S, Hiyamuta E, Arahata K, Sugita H (1991) Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve 14(6):515–520
Pastoret C, Sebille A (1995) Mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci 129(2):97–105
Pierezan F, Mansell J, Ambrus A, Rodrigues Hoffmann A (2014) Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J Comp Pathol 151:347–351
Pizza FX, Koh TJ, McGregor SJ, Brooks SV (2001a) Muscle inflammatory cells after passive stretches, isometric contractions and lengthening contractions. J Appl Physiol (1985) 92(5):1873–1878
Pizza FX, McLoughlin TJ, McGregor SJ, Calomeni EP, Gunning WT (2001b) Neutrophils injure cultured skeletal myotubes. Am J Physiol Cell Physiol 281(1):C335–C341
Pizza FX, Peterson JM, Baas JH, Koh TJ (2005) Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 562(Pt 3):899–913
Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH (2002) A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 11(3):263–272
Porter JD, Merriam AP, Leahy P, Gong B, Feuerman J, Cheng G, Khanna S (2004) Temporal gene expression profiling of dystrophin-deficient (mdx) mouse diaphragm identifies conserved and muscle group-specific mechanisms in the pathogenesis of muscular dystrophy. Hum Mol Genet 13(3):257–269
Robertson TA, Maley MA, Grounds MD, Papadimitriou JM (1993) The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 207(2):321–331
Schneider JS, Shanmugam M, Gonzalez JP, Lopez H, Gordan R, Fraidenraich D, Babu GJ (2013) Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2 + uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J Muscle Res Cell Motil 34(5–6):349–356. doi:10.1007/s10974-013-9350-0
Shavlakadze T, White J, Hoh JF, Rosenthal N, Grounds MD (2004) Targeted expression of insulin-like growth factor-I reduces early myofiber necrosis in dystrophic mdx mice. Mol Ther 10(5):829–843
Snow MH (1977) Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec 188(2):201–217
Snow MH (1978) An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res 186(3):535–540
Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352(6335):536–539
Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G (2010) Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 120(1):11–19. doi:10.1172/JCI40373
Ten Broek RW, Grefte S, Von den Hoff JW (2010) Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224(1):7–16. doi:10.1002/jcp.22127
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288(2):R345–R353
Tsivitse S (2010) Notch and Wnt signaling, physiological stimuli and postnatal myogenesis. Int J Biol Sci 6(3):268–281
Walden DL, McCutchan HJ, Enquist EG, Schwappach JR, Shanley PF, Reiss OK, Terada LS, Leff JA, Repine JE (1990) Neutrophils accumulate and contribute to skeletal muscle dysfunction after ischemia-reperfusion. Am J Physiol 259(6 Pt 2):H1809–H1812
Weller B, Karpati G, Carpenter S (1990) Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci 100(1–2):9–13
Zacks SI, Sheff MF (1982) Age-related impeded regeneration of mouse minced anterior tibial muscle. Muscle Nerve 5(2):152–161
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number 15J01551.
Authors’ contributions
YK conceptualized the study. TI, OI, TN, YE, and YK designed the experiments. TI performed the experiments and analyzed the data. TI, OI, and YK drafted the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest
Rights and permissions
About this article
Cite this article
Ikeda, T., Ichii, O., Otsuka-Kanazawa, S. et al. Degenerative and regenerative features of myofibers differ among skeletal muscles in a murine model of muscular dystrophy. J Muscle Res Cell Motil 37, 153–164 (2016). https://doi.org/10.1007/s10974-016-9452-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-016-9452-6