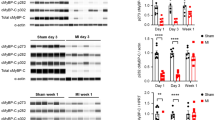
Abstract
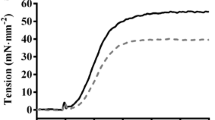
Cardiac myosin binding protein-C (cMyBP-C) plays a role in sarcomeric structure and stability, as well as modulating heart muscle contraction. The 150 kDa full-length (FL) cMyBP-C has been shown to undergo proteolytic cleavage during ischemia–reperfusion injury, producing an N-terminal 40 kDa fragment (mass 29 kDa) that is predominantly associated with post-ischemic contractile dysfunction. Thus far, the pathogenic properties of such truncated cMyBP-C proteins have not been elucidated. In the present study, we hypothesized that the presence of these 40 kDa fragments is toxic to cardiomyocytes, compared to the 110 kDa C-terminal fragment and FL cMyBP-C. To test this hypothesis, we infected neonatal rat ventricular cardiomyocytes and adult rabbit ventricular cardiomyocytes with adenoviruses expressing the FL, 110 and 40 kDa fragments of cMyBP-C, and measured cytotoxicity, Ca2+ transients, contractility, and protein–protein interactions. Here we show that expression of 40 kDa fragments in neonatal rat ventricular cardiomyocytes significantly increases LDH release and caspase 3 activity, significantly reduces cell viability, and impairs Ca2+ handling. Adult cardiomyocytes expressing 40 kDa fragments exhibited similar impairment of Ca2+ handling along with a significant reduction of sarcomere length shortening, relaxation velocity, and contraction velocity. Pull-down assays using recombinant proteins showed that the 40 kDa fragment binds significantly to sarcomeric actin, comparable to C0–C2 domains. In addition, we discovered several acetylation sites within the 40 kDa fragment that could potentially affect actomyosin function. Altogether, our data demonstrate that the 40 kDa cleavage fragments of cMyBP-C are toxic to cardiomyocytes and significantly impair contractility and Ca2+ handling via inhibition of actomyosin function. By elucidating the deleterious effects of endogenously expressed cMyBP-C N-terminal fragments on sarcomere function, these data contribute to the understanding of contractile dysfunction following myocardial injury.








Similar content being viewed by others
Abbreviations
- cMyBP-C:
-
Cardiac myosin binding protein-C
- Ca2+ :
-
Calcium ions
- h:
-
Hour(s)
- I–R:
-
Ischemia–reperfusion
- Ig:
-
Immunoglobulin
- kDa:
-
Kilodaltons
- LDH:
-
Lactate dehydrogenase
- MI:
-
Myocardial infarction
- M domain:
-
Myosin binding domain
- Min:
-
Minutes
- MOI:
-
Multiplicity of infection
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- N′:
-
N-terminal
- NRVCM:
-
Neonatal rat ventricular cardiomyocyte
- PBS:
-
Phosphate buffered saline
- PKA:
-
Protein kinase A
- SR:
-
Sarcoplasmic reticulum
References
Barefield D, Sadayappan S (2010) Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48:866–875
Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185:1083–1095
Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ (2002) Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 99:6252–6256
Copeland O, Sadayappan S, Messer AE, Steinen GJ, van der Velden J, Marston SB (2010) Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol 49:1003–1011
Decker RS, Decker ML, Kulikovskaya I, Nakamura S, Lee DC, Harris K, Klocke FJ, Winegrad S (2005) Myosin-binding protein C phosphorylation, myofibril structure, and contractile function during low-flow ischemia. Circulation 111:906–912
Freiburg A, Gautel M (1996) A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem 235:317–323
Ge Y, Rybakova IN, Xu Q, Moss RL (2009) Top-down high-resolution mass spectrometry of cardiac myosin binding protein C revealed that truncation alters protein phosphorylation state. Proc Natl Acad Sci USA 106:12658–12663
Gilbert R, Cohen JA, Pardo S, Basu A, Fischman DA (1999) Identification of the A-band localization domain of myosin binding proteins C and H (MyBP-C, MyBP-H) in skeletal muscle. J Cell Sci 112(Pt 1):69–79
Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S (2012) Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol 52:154–164
Gruen M, Gautel M (1999) Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol 286:933–949
Gruen M, Prinz H, Gautel M (1999) cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett 453:254–259
Gupta MP, Samant SA, Smith SH, Shroff SG (2008) HDAC4 and PCAF bind to cardiac sarcomeres and play a role in regulating myofilament contractile activity. J Biol Chem 283:10135–10146
Haim TE, Dowell C, Diamanti T, Scheuer J, Tardiff JC (2007) Independent FHC-related cardiac troponin T mutations exhibit specific alterations in myocellular contractility and calcium kinetics. J Mol Cell Cardiol 42:1098–1110
Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL (2002) Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res 90:594–601
Harris SP, Rostkova E, Gautel M, Moss RL (2004) Binding of myosin binding protein-C to myosin subfragment S2 affects contractility independent of a tether mechanism. Circ Res 95:930–936
Herron TJ, Rostkova E, Kunst G, Chaturvedi R, Gautel M, Kentish JC (2006) Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ Res 98:1290–1298
Howarth JW, Ramisetti S, Nolan K, Sadayappan S, Rosevear PR (2012) Structural insight into unique cardiac myosin-binding protein-C motif: a partially folded domain. J Biol Chem 287:8254–8262
Jeong EM, Wang X, Xu K, Hossain MM, Jin JP (2009) Nonmyofilament-associated troponin T fragments induce apoptosis. Am J Physiol Heart Circ Physiol 297:H283–H292
Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H (2006) Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113:51–59
Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23:607–618
Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, Morad M (2003) Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res 92:428–436
Kulikovskaya I, McClellan G, Flavigny J, Carrier L, Winegrad S (2003) Effect of MyBP-C binding to actin on contractility in heart muscle. J Gen Physiol 122:761–774
Luther PK, Winkler H, Taylor K, Zoghbi ME, Craig R, Padron R, Squire JM, Liu J (2011) Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc Natl Acad Sci USA 108:11423–11428
McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, Niimura H, Schoen FJ, Conner D, Fischman DA, Seidman CE, Seidman JG (1999) Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest 104:1235–1244
Mun JY, Gulick J, Robbins J, Woodhead J, Lehman W, Craig R (2011) Electron microscopy and 3D reconstruction of F-actin decorated with cardiac myosin-binding protein C (cMyBP-C). J Mol Biol 410:214–225
O’Brien PJ (2006) Blood cardiac troponin in toxic myocardial injury: archetype of a translational safety biomarker. Expert Rev Mol Diagn 6:685–702
Ratti J, Rostkova E, Gautel M, Pfuhl M (2011) Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem 286:12650–12658
Rybakova IN, Greaser ML, Moss RL (2011) Myosin binding protein C interaction with actin: characterization and mapping of the binding site. J Biol Chem 286:2008–2016
Sadayappan S (2012) Cardiac myosin binding protein-C: a potential early-stage, cardiac-specific biomarker of ischemia–reperfusion injury. Biomark Med 6:69–72
Sadayappan S, de Tombe P (2012) Cardiac myosin binding protein-C: redefining its structure and function. Biophys Rev. doi:10.1007/s12551-012-0067-x
Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J (2005) Cardiac myosin binding protein-C phosphorylation and cardiac function. Circ Res 97:1156–1163
Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J (2006) Cardiac myosin binding protein-C phosphorylation is cardioprotective. Proc Natl Acad Sci USA 103:16918–16923
Sadayappan S, Greis KD, Robbins J (2008) Phosphorylation-dependent proteolysis and pathogenesis of cardiac myosin binding protein-C. J Mol Cell Cardiol 44:S44
Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Robbins J (2009) Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background. Circulation 119:1253–1262
Sadayappan S, Gulick J, Osinska H, Barefield D, Cuello F, Avkiran M, Lasko VM, Lorenz JN, Maillet M, Martin JL, Brown JH, Bers DM, Molkentin JD, James J, Robbins J (2011) A critical function for ser-282 in cardiac myosin binding protein-C phosphorylation and cardiac function. Circ Res 109:141–150
Sarkey JP, Chu M, McShane M, Bovo E, Mou YA, Zima AV, de Tombe PP, Kartje GL, Martin JL (2011) Nogo-A knockdown inhibits hypoxia/reoxygenation-induced activation of mitochondrial-dependent apoptosis in cardiomyocytes. J Mol Cell Cardiol 50:1044–1055
Shaffer JF, Kensler RW, Harris SP (2009) The myosin binding protein-C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem 284:12318–12327
Shaffer JF, Wong P, Bezold KL, Harris SP (2010) Functional differences between the N-terminal domains of mouse and human myosin binding protein-C. J Biomed Biotechnol 2010:789798
Squire JM, Luther PK, Knupp C (2003) Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol 331:713–724
Weith A, Sadayappan S, Gulick J, Previs MJ, Vanburen P, Robbins J, Warshaw DM (2012) Unique single molecule binding of cardiac myosin binding protein-C to actin and phosphorylation-dependent inhibition of actomyosin motility requires 17 amino acids of the motif domain. J Mol Cell Cardiol 52:219–227
Winegrad S (1999) Cardiac myosin binding protein C. Circ Res 84:1117–1126
Acknowledgments
We thank our collaborators, Jody L. Martin, PhD, in the adenoviral core faculty for viral production and NRVCMs preparation, and Aleksey Zima, PhD, for providing us the rabbit adult ventricular cardiomyocytes at the Cell and Molecular Physiology, Loyola University Chicago, Stritch School of Medicine, Maywood, IL 60153, USA. We also thank, Jeffery D. Molkentin, PhD, Cincinnati Children’s Hospital, Cincinnati, OH 45229, USA, for providing us the mouse I–R injury surgery training.
Funding
This study was funded by National Institutes of Health grants HL007692 (Dr. Sarkey), HL101297, HL62426 (Dr. de Tombe) and R01HL105826 (Dr. Sadayappan), American Heart Association—Post-Doctoral Training Grant (10POST4230040 to Dr. Sundaresan) and—Scientist Development Grant (0830311N to Dr. Sadayappan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Suresh Govindan and Jason Sarkey contributed equally to this work.
Rights and permissions
About this article
Cite this article
Govindan, S., Sarkey, J., Ji, X. et al. Pathogenic properties of the N-terminal region of cardiac myosin binding protein-C in vitro. J Muscle Res Cell Motil 33, 17–30 (2012). https://doi.org/10.1007/s10974-012-9292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-012-9292-y