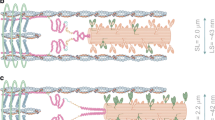
Abstract
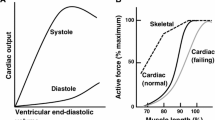
The steep relationship between systolic force and end diastolic volume in cardiac muscle (Frank–Starling relation) is, to a large extent, based on length-dependent changes in myofilament Ca2+ sensitivity. How sarcomere length modulates Ca2+ sensitivity is still a topic of active investigation. Two general themes have emerged in recent years. On the one hand, there is a large body of evidence indicating that length-dependent changes in lattice spacing determine changes in Ca2+ sensitivity for a given set of conditions. A model has been put forward in which the number of strong-binding cross-bridges that are formed is directly related to the proximity of the myosin heads to binding sites on actin. On the other hand, there is also a body of evidence suggesting that lattice spacing and Ca2+ sensitivity are not tightly linked and that there is a length-sensing element in the sarcomere, which can modulate actin–myosin interactions independent of changes in lattice spacing. In this review, we examine the evidence that has been cited in support of these viewpoints. Much recent progress has been based on the combination of mechanical measurements with X-ray diffraction analysis of lattice spacing and cross-bridge interaction with actin. Compelling evidence indicates that the relationship between sarcomere length and lattice spacing is influenced by the elastic properties of titin and that changes in lattice spacing directly modulate cross-bridge interactions with thin filaments. However, there is also evidence that the precise relationship between Ca2+ sensitivity and lattice spacing can be altered by changes in protein isoform expression, protein phosphorylation, modifiers of cross-bridge kinetics, and changes in titin compliance. Hence although there is no unique relationship between Ca2+ sensitivity and lattice spacing the evidence strongly suggests that under any given set of physiological circumstances variation in lattice spacing is the major determinant of length-dependent changes in Ca2+ sensitivity.
Similar content being viewed by others
References
Adhikari BB, Fajer PG, (1996) Myosin head orientation and mobility during isometric contraction: effects of osmotic compression Biophys J 70: 1872–1880
Adhikari B, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA, (2004) Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling Biophys J 87: 1784–1794
Allen DG, Jewell BR, Murray JW, (1974) The contribution of activation process to the length-tension relation in cardiac muscle Nature 248: 509–513
Allen DG, Kentish JC, (1985) The cellular basis of the length-tension relation in cardiac muscle J Mol Cell Cardiol 17: 821–840
Allen DG, Kentish JC, (1988) Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length J Physiol 407:489–503
Allen DG, Kurihara S, (1982) The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle J Physiol 327: 79–94
Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ, (2000) Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I J Physiol 526: 541–549
Balnave CD, Allen DG, (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle J Physiol 492: 705–713
Bremel RD, Weber A, (1972) Cooperation within actin filament in striated muscle Nature New Biol 238: 97–101
Brenner B, Yu LC, (1985) Equatorial X-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation Biophys J 48: 829–834
Brenner B, Yu LC, Chalovich JM, (1991) Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon Proc Nat Acad Sci USA 88: 5739–5743
Brenner B, Yu LC, Podolsky RJ, (1984) X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths Biophys J 46: 299–306
Cazorla O, Pascarel C, Garnier D, LeGuennec J-Y, (1997) Resting tension participates in the modulation of active tension in isolated guinea pig ventricular myocytes J Mol Cell Cardiol 29: 1629–1637
Cazorla O, Vassort G, Garnier D, LeGuennec J-Y, (1999) Length modulation of active force in rat cardiac myocytes: is titin the sensor? J Mol Cell Cardiol 31: 1215–1227
Cazorla O, Wu Y, Irving TC, Granzier H, (2001) Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes Circ Res 88: 1028–1035
Close RI, (1972) The relations between sarcomere length and characteristics of isometric twitch contractions of frog sartorius muscle J Physiol 220: 745–762
Endo M, (1972) Stretch-induced increase in activation of skinned muscle fibres by calcium Nature New Biol 237: 211–213
Fabiato A, (1981) Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle J Gen Physiol 78: 457–497
Fabiato A, Fabiato F, (1978) Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells J Gen Physiol 72: 677–699
Fitzsimons DP, Moss RL, (1998) Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes Circ Res 83: 602–607
Fuchs F, (1995) Mechanical modulation of the Ca2+ regulatory protein complex in cardiac muscle News Physiol Sci 10:6–12
Fuchs F, (2002) The Frank–Starling relationship: cellular and molecular mechanisms. In: Solaro RJ, Moss RL, (eds.) Molecular Control Mechanisms in Striated Muscle Contraction. Kluwer, Dordrecht, pp. 379–415
Fuchs F, Smith SH, (2001) Calcium, cross-bridges, and the Frank–Starling relationship News Physiol Sci 16: 5–10
Fuchs F, Wang YP, (1991) Force, length, and Ca2+-troponin C affinity in skeletal muscle Am J Physiol 261: C787–C792
Fuchs F, Wang Y, (1996) Sarcomere length vs. interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding J Mol Cell Cardiol 28: 1375–1383
Fujino K, Sperelakis N, Solaro RJ, (1988) Sensitization of dog and guinea pig heart myofilaments to Ca2+ activation and the inotropic effect of pimobendan: comparison with milrinone Circ Res 63: 911–922
Fujita H, Labeit D, Gerull B, Labeit S, Granzier HL, (2004) Titin isoform-dependent effect of calcium on passive myocardial tension Am J Physiol 287: H2528–H2534
Fukuda N, Kajiwara H, Ishiwata S, Kurihara S, (2000) Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle Circ Res 86: e1–e6
Fukuda N, Jin O-U, Sasaki D, Kajiwara H, Ishiwata S, Kurihara S, (2001a) Acidosis or inorganic phosphate enhances the length dependence of tension in rat skinned cardiac muscle J Physiol 536: 153–160
Fukuda N, Sasaki D, Ishiwata S, Kurihara S, (2001b) Length dependence of tension generation in rat skinned cardiac muscle. Role of titin in the Frank–Starling mechanism of the heart Circulation 104:1639–1645
Fukuda N, Wu Y, Irving TC, Granzier H, (2003) Titin isoform variance and length dependence of activation in skinned bovine cardiac muscleJ Physiol 553:147–154
Fukuda N, Wu Y, Farman G, Irving TC, Granzier H, (2005a) Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle Pflugers Arch 449: 449–457
Fukuda N, Wu Y, Nair P, Granzier HL, (2005b) Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner J Gen Physiol 124: 257–271
Gao WD, Backx PH, Azan-Backx M, Marban E, (1994) Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle Circ Res 74: 408–415
Godt RE, Maughan DW, (1977) Swelling of skinned muscle fibres of the frog Biophys J 19: 103–116
Godt RE, Maughan DW, (1981) Influence of osmotic compression on calcium activation and tension in skinned muscle fibres of the rabbit Pflugers Arch 391: 334–337
Gordon AM, Homsher E, Regnier M, (2000) Regulation of contraction in striated muscle Physiol Rev 80: 853–924
Gordon AM, Huxley AF, Julian FJ, (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres J Physiol 184: 170–192
Granzier H, Labeit S, (2002) Cardiac titin: an adjustable multifunctional spring J Physiol 541: 335–342
Harrison SM, Lamont C, Miller DJ, (1988) Hysteresis and the length dependence of calcium sensitivity in chemically-skinned rat cardiac muscle J Physiol 401:115–143
Head JG, Ritchie MD, Geeves MA, (1995) Characterization of the equilibrium between blocked and closed states of muscle thin filaments Eur J Biochem 227: 694–699
Hibberd MJ and Jewell BR (1982) Calcium and length dependent force production in rat ventricular muscle. J Physiol 329: 527–540.
Hofmann PA, Fuchs F, (1987a) Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C Am J Physiol 253: C90–C96
Hofmann PA, Fuchs F, (1987b) Evidence for a force-dependent component of calcium binding to cardiac troponin C Am J Physiol 253: C541–C546
Hofmann PA, Fuchs F, (1988) Bound calcium and force development in skinned cardiac muscle bundles: effect of sarcomere length J Mol Cell Cardiol 20: 667–677
Irving TC, Konhilas J, Perry D, Fischetti R, DeTombe PP, (2000) Myofilament lattice spacing as a function of sarcomere length in isolated myocardium Am J Physiol 279: H2568–H2573
Jewell BR, (1977) A reexamination of the influence of muscle length on cardiac performance Circ Res 40: 221–230
Kajiwara H, Morimoto S, Fukuda N, Ohtsuki I, Kurihara S, (2000) Effect of troponin I phosphorylation by protein kinase A on length-dependence of tension activation in skinned cardiac muscle fibers Biochem Biophys Res Comm 272: 104–110
Kawai M, Wray JS, Zhao Y, (1993) The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. I. proportionality between the lattice spacing and the fiber width Biophys J 64: 187–196
Kentish JC, ter Keurs HEDJ, Ricciardi L, Bucx JJJ, Noble MIM, (1986) Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle: influence of calcium concentrations on these relations Circ Res 58: 755–768
Komukai K, Kurihara S, (1997) Length dependence of Ca2+–tension relationship in aequorin-injected ferret papillary muscles Am J Physiol 273: H1068–H1074
Konhilas JP, Irving TC, deTombe PP, (2002a) Frank–Starling law of the heart and the cellular mechanisms of length-dependent activation Pflugers Arch 445:305–310
Konhilas JP, Irving TC, deTombe PP, (2002b) Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing Circ Res 90: 59–65
Konhilas JP, Irving TC, deTombe PP, (2002c) Length-dependent activation in three striated muscle types of the rat J Physiol 544: 225–236
Konhilas JP, Irving TC, Wolska BM, Jwied EE, Martin AF, Solaro RJ, deTombe PP, (2003) Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing J Physiol 537: 951–961
Kraft T, Brenner B, (1997) Force enhancement without changes in cross-bridge turnover kinetics: the effect of EMD 57033 Biophys J 72: 272–282
Lakatta EG, (1992) Length modulation of muscle performance: Frank–Starling law of the heart. In: Fozzard HA, Jennings RB, Haber E, Katz AM, Morgan HA, (eds.). The Heart and Cardiovascular System Vol. II. Raven Press, New York, pp.1325–1351
Lakatta EG, Jewell BR, (1977) Length-dependent activation. Its’effect on the length-tension relation in cat ventricular muscle Circ Res 40: 251–257
Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyck JE, Tobacman LS, Craig R, (2000) Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments J Mol Biol 302: 593–606
Lehrer SS, (1994) The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil 15:232–236
Levine RJC, Kensler RW, Yang Z, Stull JT, Sweeney HL, (1996) Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments Biophys J 71: 898–907
Martyn DA, Gordon AM, (1988) Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength J Muscle Res Cell Motil 9:428–445
Martyn DA, Gordon AM, (2001) Influence of length or force on activation-dependent changes in troponin C structure in skinned cardiac and fast skeletal muscle Biophys J 80: 2798–2808
Martyn DA, Regnier M, Xu D, Gordon AM, (2001) Ca2+- and cross-bridge-dependent changes in N- and C- terminal structure of troponin C in rat cardiac muscle Biophys J 80: 360–370
Martyn DA, Adhikari BB, Regnier M, Gu J, Xu S, Yu LC, (2004) Response of equatorial X-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle Biophys J 86: 1002–1011
Martyn DA, Smith L, (2005a) The temperature dependence of length-dependent contractile activation in cardiac muscle Biophys J 88: 120a
Martyn DA, Smith L, (2005b) The length sensitivity of contractile activation in cardiac muscle is dependent on the extent of strong CB binding Biophys J 88: 121a
McDonald KS, Moss RL, (1995) Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length Circ Res 77: 199–205
McKillop DFA, Geeves MA, (1993) Regulation of the interaction between actin and myosin subfragmant 1: evidence for three states of the thin filament Biophys J 65: 693–701
Metzger J, (1995) Myosin binding-induced cooperative activation of the thin filament in cardiac myocytes and skeletal muscle fibers Biophys J 68: 1430–1442
Millman BM, (1998) The filament lattice of striated muscle Physiol Rev 78: 359–391
Muhle-Goll C, Habeck M, Cazorla O, Nilges M, Labeit, Granzier H, (2001) Structural and functional studies of titin’s fn3 modules reveal conserved surface patterns and binding to myosin S1–a possible role in the Frank–Starling mechanism of the heart J Mol Biol 313: 431–447
Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL, (2004) Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity and force and activation dependence of the kinetics of myocardial force development Am J Physiol 287: H2712–H2718
Patel JR, McDonald KS, Wolff MR, Moss RL, (1997) Ca2+ binding to troponin C in skinned skeletal muscle fibers assessed with caged Ca2+ and a Ca2+ fluophore: invariance of Ca2+ binding as a function of sarcomere length J Biol Chem 272: 6018–6027
Pirani A, Xu C, Hatch V, Craig R, Tobacman LS, Lehman W, (2005) Single particle analysis of relaxed and activated muscle thin filaments J Mol Biol 346:761–772
Regnier M, Rivera AJ, Wang C-K, Bates MA, Chase PB, Gordon AM, (2002) Thin filament near-neighbor regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca2+ relations J Physiol 540: 485–497
Rice JJ, deTombe PP, (2004) Approaches to modeling cross-bridges and calcium-dependent activation in cardiac muscle Prog Biophys Mol Biol 85: 179–195
Robinson JM, Dong W-J, Xing J, Cheung HC, (2004) Switching of troponin I: Ca2+ and myosin induced activation of heart muscle J Mol Biol 340: 295–305
Saeki Y, Kurihara S, Hongo K, Tanaka E, (1993) Alterations in intracellular calcium and tension of activated ferret papillary muscle in response to step length changes J Physiol 463: 291–306
Smith SH, Fuchs F, (1999) Effect of ionic strength on length-dependent Ca2+ activation in skinned cardiac muscle J Mol Cell Cardiol 31:2115–2125
Smith SH, Fuchs F, (2000) Length-dependence of cross-bridge mediated activation of the cardiac thin filament J Mol Cell Cardiol 32: 831–838
Smith SH, Fuchs F, (2002) Length dependence of cardiac myofilament Ca2+ sensitivity in the presence of substitute nucleoside triphosphates J Mol Cell Cardiol 34:547–554
Stephenson DG, Wendt IR, (1984) Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres J Musc Res Cell Motil 5: 243–272
Stienen GJM, Blange T, Treijtel BW, (1985) Tension development and calcium sensitivity in skinned muscle fibers of the frog Pflugers Arch 405: 19–23
ter Keurs HEDJ, Rignsburger WH, van Heuningen R, Nagelsmit MJ, (1980) Tension development and sarcomere length in rat cardiac trabeculae: evidence of length-dependent activation Circ Res 46: 703–714
Tskhovrebova L, Trinick J, (2003) Titin: properties and family relationships Nat Rev Mol Cell Biol 4: 679–689
van der Velden J, deJong JW, Owen VJ, BurtonPBJ, Stienen GJM, (2000) Effect of protein kinase A on calcium sensitivity of force and its’ sarcomere length dependence in human cardiomyocytes Cardiovasc Res 46: 487–495
van der Velden J, Papp Z, Zaremba R, Boontje NM, deJong JW, Owen VJ, Burton PBJ, Goldmann P, Jaquet K, Stienen GJM, (2003). Increased Ca2+ sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins Cardiovasc Res 57: 37–47
Vibert P, Craig R, Lehman W, (1997) Steric-model for activation of muscle thin filaments J Mol Biol 266: 8–14
Wang Y-P, Fuchs F, (1994) Length, force, and Ca2+-troponin C affinity in cardiac and slow skeletal muscle Am J Physiol 266: C1077–C1082
Wang Y, Fuchs F, (1995) Osmotic compression of skinned cardiac and skeletal muscle bundles: effects on force generation, Ca2+ sensitivity, and Ca 2+ binding J Mol Cell Cardiol 27: 1235–1244
Wang Y, Fuchs F, (2001) Interfilament spacing, Ca2+ sensitivity, and Ca2+ binding in skinned bovine cardiac muscle J Muscle Res Cell Motil 22: 251–257
Wolska BM, Kitada Y, Palmiter KA, Westfall MV, Johnson MD, Solaro RJ, (1996) CGP-48506 increases contractility of ventricular myocytes and myofilaments by effects on actin–myosin reaction Am J Physiol 270: H24–H32
Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H, (2002) Protein kinase A phosphorylates titin’s cardiac specific N2B domain and reduces passive tension in rat cardiac myocytes Circ Res 90: 1181–1188
Yagi N, Shimizu J, Mohri S, Araki J, Nakamura K, Okuyama H, Toyota H, Morimoto T, Morizane Y, Kurusu M, Hashimoto K, Tsujioka K, Suga H, Kaliya F, (2004) X-ray diffraction from a left ventricular wall of rat heart Biophys J 86: 2286–2294
Yang Z, Stull JT, Levine RJC, Sweeney HL, (1998) Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers J Struct Biol 122: 139–148
Zuurbier CJ, Lee-de Groot MBE, Van der Laarse WJ, Huijing PA, (1998) Effects of in vivo-like activation frequency on the length-dependent force generation of skeletal muscle fiber bundles Eur J Appl Physiol 77: 503–510
Acknowledgements
The authors would like to thank Dr Albert M. Gordon for his reading of this manuscript and helpful, insightful comments. We also thank Dr Paolo Vicini for his comments on statistical treatment of force–pCa data.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Some of the papers cited in this review raise the question of whether there is a need for the muscle research community to arrive at some consensus as to the most appropriate means to quantitate change in Ca2+ sensitivity. In the case of skinned fiber studies the great majority of investigators have expressed Ca2+ sensitivity in terms of the pCa at which normalized force is half-maximal (pCa50); the difference at, for example, two different lengths would be ΔpCa50. In the papers of Konhilas et al., Ca2+ sensitivity is expressed in terms of the absolute Ca2+ concentration needed for half maximal force (EC50; μM) and the length-related difference is then ΔEC50. While one would hope to arrive at the same qualitative answer regardless of which measure is used, in actual practice this is not always the case, as is seen in the paper on ssTnI substitution and PKA phosphorylation (Konhilas et al., 2003). With ssTnI substitution the ΔEC50 changed from 0.41 to 0.26, a statistically significant difference, but the ΔpCa50 did not change at all (0.12 vs. 0.13). With PKA treatment of wild type fibers the ΔEC50 was increased from 0.41 to 0.79 but the ΔpCa50 was the same (0.12) for both phosphorylated and non-phosphorylated fibers. In the paper comparing cardiac, and fast and slow skeletal muscle (Konhilas et al., 2002c) the ΔEC50 values followed the order 0.65 (cardiac), 0.42 (fast skeletal), and 0.25 (slow skeletal). The ΔpCa50 values followed the order 0.09 (cardiac), 0.08 (fast skeletal), and 0.05 (slow skeletal). That is, by one measure cardiac muscle had greater length sensitivity than fast skeletal muscle but by another measure there was no discernible difference.
One consideration when comparing EC50 (absolute [Ca2+]) and pCa50 (the –log [Ca2+]) may be the question of which parameter exhibits a Gaussian (normal) or non-Gaussian distribution in a population of fibers. When either parameter is estimated by fitting the population data with the Hill equation (as in Figure 1) the estimate of fit is normally distributed and is characterized by an estimated mean value and the corresponding standard error. In contrast, a histogram of EC50 or pCa50 values from fits to force-[Ca2+] data from several individual fibers may or may not be normally distributed, raising the question of which is the preferred characterization; to our knowledge this comparison has not appeared in the literature. On the other hand, even if (as could be expected) either EC50 or pCa50 exhibits a normal distribution and the other does not, the central tendencies of both can be recovered through an appropriate statistical estimate which would be normally distributed. It is not clear that these considerations would influence the conclusions regarding the effects of length on EC50 or pCa50 described above.
Which is the more valid measure of length sensitivity, ΔEC50 or ΔpCa50? An advantage of ΔpCa50 as the preferred measure is that its value is independent of where the [Ca2+] lies on the absolute scale. To take an admittedly extreme case, let us suppose that Muscle A has EC50 values at long and short sarcomere length of 1.0 and 2.0 μM, respectively, and Muscle B has corresponding values of 10 and 20 μM. The ΔEC50 values differ by a factor of 10 but the ΔpCa50 value in both cases is 0.3, reflecting the fact that in each case the ratio of EC50 at the two lengths is identical. In both cases an equivalent degree of stretch produces a two-fold increase in Ca2+ sensitivity. The question then becomes one of absolute change versus relative change and which one is more physiologically meaningful. Put another way, a significant difference in a pair of ΔpCa50 values will always be associated with a significant difference in ΔEC50, but the converse is not always true. Thus while both measures may have a legitimate use it could be argued that the ΔpCa50 provides a less ambiguous measure of a change in sensitivity.
Rights and permissions
About this article
Cite this article
Fuchs, F., Martyn, D.A. Length-dependent Ca2+ activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil 26, 199–212 (2005). https://doi.org/10.1007/s10974-005-9011-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-005-9011-z