Abstract
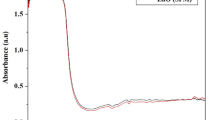
In current study, thermal and other analytical techniques have been successfully utilized in characterization of bifenthrin urea co-inclusion complex (BNUCIC)—a human-guarded insecticide formulation. Bifenthrin (BN)—a widely used pyrethroid insecticide was successfully engulfed in the cavities of hexagonal urea lattice in the presence of a suitable rapidly complexing agent (RCA). Resulting formulation shields human beings from insecticides through direct inhalation, ingestion or dermal contact. Insects will be exposed to insecticide only after BNUCIC comes in contact with water in soil/crops following switching on of water sprinkling/distribution system in the fields. Formation of BNUCIC was characterized by DSC, FTIR, XRD and 1H-NMR studies. Thermal analysis played a key role in characterization, in estimation of the minimum proportion of RCA needed for complexation and to study the influence of relative proportion of RCA on heat of decomposition of complexes. Thermal analysis depicted gradual increase in heat of decomposition of BNUCIC and excellent r 2 value with increasing molar fraction of linear chain RCA. Increased heat of decomposition ensures improved physical stability of complexes. Overlay of DSC curves of BNUCIC complexes revealed absence of melting endotherm of insecticide indicating amorphous nature of BN. FTIR spectrum and XRD diffractogram depicted characteristic peaks and interplanar spacings of hexagonal urea. 1H-NMR spectrum revealed presence of exposed protons of guest moieties in hexagonal urea. BNUCIC exhibited uniform formulation composition and improved dissolution profile. Studies reveal insecticide–fertilizer amalgamation to be a useful technique for formulation of an effective human-guarded insecticide formulation with improved characteristics.









Similar content being viewed by others
References
Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues and risk assessment indicators. Int J Environ Res Public Health. 2011;. doi:10.3390/ijerph8051402.
Gilden RC, Huffling K, Sattler B. Pesticides and health risks. J Obstet Gynecol. 2010;39(1):103–10.
Class TJ, Kintrup J. Pyrethroids as household insecticides: analysis, indoor exposure and persistence. Fresenius J Anal Chem. 1991;. doi:10.1007/BF00322420.
Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS, Symington S, Clark JM, Burr S, Ray D. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology. 2009;. doi:10.1016/j.neuro.2009.09.002.
Pranschke AM, Hooper BLM, Moser B. Efficacy of bifenthrin in treatment zones against red imported fire ant. J Econ Entomol. 2003;96(1):98–105.
Hougard JM, Zaim SD, Guillet P. Bifenthrin: a useful pyrethroid insecticide for treatment of mosquito nets. J Med Entomol. 2002;39(3):526–33.
Chen S, Luo J, Hu M, Geng P, Zhang Y. Microbial detoxication of bifenthrin by a novel yeast and its potential for contaminated soils treatment. PLoS ONE. 2012;. doi:10.1371/journal.pone.0030862.
Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30:55–78.
Weston DP, Holmes RW, You J, Lydy JM. Aquatic toxicity due to residential use of pyrethroid insecticides. Environ Sci Technol. 2005;39:9778–84.
World Health Organization. The WHO recommended classification of pesticides by hazard and guidelines to classification (2009) (Report) World Health Organization. Accessed 14 May 2015.
US EPA. Office of pesticide programs list of chemicals evaluated for carcinogenic potential. Memo from WL Burnman, HED, to HED branch chiefs. Washington. February 19, 1997.
US Environmental Protection Agency. (US EPA). Bifenthrin, pesticide tolerance. Fed Reg. 2003;68(83):28056–68.
Wang LM, Liu WP, Yang CX, Pan ZY, Gan JY. Enantioselectivity in estrogenic potential and uptake of bifenthrin. Environ Sci Technol. 2007;41:6124–8.
Connors SL, Levitt P, Matthews SG, Slotkin TA, Johnston MV, Kinney HC, Johnson WG, Dailey RM, Zimmerman AW. Fetal mechanisms in neurodevelopmental disorders. Paedia Neurol. 2008;. doi:10.1016/j.pediatrneurol.2007.10.009.
Lawkowski DA. Physical and chemical properties of pyrethroids. Rev Environ Contam. 2002;T174:49–170.
Yi-Jun H, Yan XU, Chao MA, Qi-Fu X, Xue-Min W. Preparation and properties of an inclusion compound of β-cyclodextrin with bifenthrin. Chin J Pestic Sci. 2010;3:324–8.
Piccolo O, Delogu G, Borzatta V. Formulation of a synergistic insecticidal composition as a cyclodextrin-complex. WO 2005039287 A2, 2005.
Nishiguchi N, Ikari K. Solid dispersions of isoxazoline compounds. EP2865369 A1, 2015.
Jorgenson BC, Young TM. Formulation effects and the off-target transport of pyrethroid insecticides from urban hard surfaces. Environ Sci Technol. 2010;44(13):4951–7.
Norton, D. Pyrethroid formulations WO 2013041975 A2, 2013.
Madan AK. Microencapsulation of low dose drugs. Ph.D. Thesis, IIT Delhi, 1994.
Madan AK, Grover PD. A process for preparation of urea based inclusion compounds of vitamin A esters. Indian Patent 180627 filed on 20/01/1993.
Madan AK, Bajaj V. A process for preparation of urea based inclusion compounds of vitamin E and its esters. Indian Patent 182620 filed on 24/10/1994.
Univar USA, Inc. Material Safety Data Sheet. Masterline bifenthrin 7.9 termiticide/insecticide. EPA Reg. No. 73748-7, 2008. pp. 1–7.
Pineiro A, Banquy X, Perez-Casas S, Tovar E, García A, Villa A, Amigo A, Mark AE, Costas M. On the characterization of Host-Guest complexes: surface tension, calorimetry, and molecular dynamics of cyclodextrins with a non-ionic surfactant. J Phys Chem. 2007;. doi:10.1021/jp0688815.
Frank SG. Inclusion compounds. J Pharm Sci. 1975;64:1585–604.
U.S. EPA (Environmental Protection Agency). Toxicological review of urea. (CAS No. 57-13-6). In support of summary information on the integrated risk information system (IRIS). External peer review draft, Washington; EPA/635/R-10/005A. http://www.epa.gov/iris/backgrd.html (September 2010). Accessed 15 June 2014.
Bengen MF. Urea channel inclusion compounds. German Patent Application OZ 123438 filed on 18/03/1940.
Marsh KL, Sims GK, Mulvaney RL. Availability of urea to autotrophic ammonia-oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil. Biol Fertil Soils. 2005;42:137–45.
Torello WA, Wehner DJ. Urease activity in a Kentucky bluegrass turf. Agron J. 1983;75:654–6.
Meessen JH, Petersen H. Urea. In: Ullmann’s encyclopedia of industrial chemistry: Wiley-VCH, Weinheim 2005; doi: 10.1002/14356007.a27_333.
Kanani-Al T, Mackenzie AF, Barhakur NN. Soil water and ammonia volatilization relationships with surface-applied nitrogen fertilizer solutions. Soil Sci Soc Am J. 1991;55:1761–6.
Harris KDM. Meldola lecture: understanding properties of urea and thiourea inclusion compounds. Chem Soc Rev. 1997;26:279–89.
Harris KDM. Fundamental and applied aspects of urea and thiourea inclusion compounds. Supramol Chem. 2007;. doi:10.1080/10610270600977706.
Rujas JM, Harris KDM, Desmedt A, Guillaume F. In-situ monitoring of alkane-alkane guest exchange in urea inclusion compounds using confocal Raman microspectrometry. Mol Cryst Liq Cryst. 2006;. doi:10.1080/15421400600788633.
Smith AE. The crystal structure of urea-hydrocarbon complexes. Acta Crystallogr. 1952;5:224–35.
Takemoto K, Sonada N. Inclusion compounds of urea, thiourea and selenourea. In: Atwood JL, Davis JED, MacNicol DD, editors. Inclusion compounds. London: Academic Press; vol. 2, 1984. pp. 47–67.
Bishop R, Dance IG. New type of helical inclusion networks. Top Curr Chem. 1988;149:139–88.
Harris KDM, Thomas JM. Structure aspects of urea inclusion compounds and their investigation by X-ray diffraction: a general discussion. J Chem Soc Faraday Trans. 1990;86:2985–96.
Smart SS, Baghdagi AE, Gullaume F. Harris KDM. Conformational and vibrational properties of α, ω-dihalogenoalkane/urea inclusion compounds: a Raman scattering investigation. J Chem Soc Faraday Trans. 1994;90:1313–22.
Hollingsworth MD, Harris KDM. Urea inclusion compounds. In: Atwood JL, Davis JED, MacNicol DD, Vogtle F, editors. Comprehensive supramolecular chemistry. Solid state supramolecular chemistry-crystal engineering, Oxford: Pergamon Press; vol. 6, 1996. pp. 177–237.
Schiessler RW, Flitter D. Urea and thiourea adduction of C5–C42–Hydrocarbons. J Am Chem Soc. 1954;74:1720–3.
Schlenk W. Urea addition of aliphatic compounds. Justus Liebigs Ann Chem. 1949;565:204–40.
Thakral S, Madan AK. Adduction of amiloride hydrochloride in urea through a modified technique for the dissolution enhancement. J Pharm Sci. 2008;97(3):1191–201.
Thakral S, Madan AK. Urea co-inclusion compounds of glipizide for the improvement of dissolution profile. J Incl Phenom Macrocycl Chem. 2008;. doi:10.1007/s10847-007-9368-2.
Thakral S, Madan AK. Urea inclusion compounds of enalapril maleate for the improvement of pharmaceutical characteristics. J Pharm Pharmacol. 2007;59(11):1501–7.
Dhall M, Madan AK. Simultaneous improvement in dissolution profile and content uniformity of lafutidine through co-inclusion in urea. J Incl Phenom Macrocycl Chem. 2015;. doi:10.1007/s10847-015-0493-z.
Dhall M, Madan AK. Studies on urea co-inclusion complexes of simvastatin for improvement of pharmaceutical characteristics. J Incl Phenom Macrocycl Chem. 2015;. doi:10.1007/s10847-014-0439-x.
Dhall M, Madan AK. Steep improvement in dissolution profile of ezetimibe through co-inclusion in urea. J Pharm Invest. 2016;. doi:10.1007/s40005-016-0236-1.
Thakral S, Madan AK. Urea co-inclusion compounds of 13 cis-retinoic acid for simultaneous improvement of dissolution profile, photostability and safe handling characteristics. J Pharm Pharmacol. 2008;60(7):823–32.
Thakral S, Madan AK. Reduction in moisture sensitivity/uptake of moisture sensitive drugs through adduction in urea. J Pharm Innov. 2008;. doi:10.1007/s12247-008-9045-z.
Dhall M, Madan AK. Urea complexes of chlorpyrifos, malathion bifenthrin and cypermethrin for improving safe handling and other characteristics. Indian Patent No. 201611002986 filed on 28/01/2016.
Dhall M, Madan AK. Conversion of viscous liquid malathion into free flowing solids through co-inclusion in urea for multiple benefits. J Incl Phenom Macrocycl Chem. 2016;. doi:10.1007/s10847-016-0648-6.
Zhang GE, Li XT, Tian SJ, Li JH, Wang JY, Lou XD, Cheng QT. Kinetic studies on the thermal dissociation of β-cyclodextrin ethyl benzoate inclusion complexes. J Therm Anal Calorim. 1998;. doi:10.1023/A:1010129028436.
Sbarcea L, Udrescu L, Ledeti I, Ledeti I, Szabadai Z, Fulias A, Sbarcea C. β-Cyclodextrin inclusion complexes of lisinopril and zofenopril. J Therm Anal Calorim. 2016;. doi:10.1007/s10973-015-5045-7.
Rocha BA, Rodrigues MR, Bueno PC, Costa-Machado AR, Vaz MM, Nascimento AP, Barud HS, Berretta-Silv AA. Preparation and thermal characterization of inclusion complex of Brazilian green propolis and hydroxypropyl-β-cyclodextrin. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1713-4.
Cloudy P, Letoffe JM, Germain P, Bastide JP, Bayol A, Blasquez S, Rao RC, Gonzalez B. Physicochemical characterization of cholesterol-beta cyclodextrin inclusion complexes. J Therm Anal Calorim. 2005;. doi:10.1007/BF01912796.
Novak CS, Ehen Z, Fodor M, Jicsinszky L, Orgovanyi J. Application of combined thermoanalytical techniques in the investigation of cyclodextrin inclusion complexes. J Therm Anal Calorim. 2006;. doi:10.1007/s10973-005-7605-8.
Aki H, Nakashima Y, Kawasaki Y, Niiya T. Thermodynamic evaluation of antibacterial activity for inclusion complexes of amoxicillin with cyclodextrins. J Therm Anal Calorim. 2006;. doi:10.1007/s10973-006-7650-y.
Benko M, Tabajdi R, Kiraly Z. Thermodynamics of formation of β-cyclodextrin inclusion complexes with four series of surfactant homologs. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-012-2603-0.
Lavor EP, Navarro MVM, Freire FD, Aragao CFS, Raffin FN, Barbosa EG, Moura TFA. Application of thermal analysis to the study of antituberculosis drugs–excipient compatibility. J Therm Anal Calorim. 2014;115(3):2303–9.
Piekarski H, Nowicika B. Calorimetric studies of interactions of some peptides with electrolytes, urea and ethanol in water at 298.15 K. J Therm Anal Calorim. 2009;. doi:10.1007/s10973-009-0547-9.
Ravindran B, Madhurambal G, Mariappan M, Mojumdar SC. Synthesis and characterization of some single crystals of thiourea urea zinc chloride. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1291-5.
Gopinath S, Barathan S, Rajesekaran R. Growth and studies of thiourea urea magnesium chloride (TUMC) single crystals. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-011-1775-3.
Madhurambal G, Mariappan M, Mojumdar SC. TG–DTA, UV and FTIR spectroscopic studies of urea–thiourea mixed crystal. J Therm Anal Calorim. 2010;. doi:10.1007/s10973-010-0763-3.
Siimer K, Christjanson P, Kaljuvee T, Pehk T, Lasn I, Saks I. TG-DTA study of melamine-urea-formaldehyde resins. J Therm Anal Calorim. 2008;. doi:10.1007/s10973-007-8721-4.
Zorba T, Papadopoulou E, Hatjissaak A, Paraskevopoulos K, Chrissafis K. Urea-formaldehyde resins characterized by thermal analysis and FTIR method. J Therm Anal Calorim. 2008;. doi:10.1007/s10973-007-8731-2.
Pumamadjaja AH, Russell RA. Pheromone communication in a robot swarm: necrophoric bee behavior and its replication. Robotica. 2005;. doi:10.1017/S0263574704001225.
Zimmerschied WJ, Dinnerstein RA, Weitkamp AW, Marschner RF. Crystalline adducts of urea with linear aliphatic compounds. Ind Eng Chem. 1950;42:1300–6.
Zhu P, Zhang G, Ma Y, Zhang Y, Miao H, Wu Y. Study of DNA interactions with bifenthrin by spectroscopic techniques and molecular modeling. Spectrochim Acta A Mol Biomol Spectrosc. 2013;. doi:10.1016/j.saa.2013.04.022.
Smith PA, Thompson MJ, Edwards JW. Estimating occupational exposure to the pyrethroid termiticide bifenthrin by measuring metabolites in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778(1–2):113–20.
US Pharmacopeia and National Formulary USP 39–NF 34. 39th ed. Rockville MD: The US Pharmacopeial Convention Inc.; 2016.
Brown WL, Policello GA. Antidrift composition. Patent application number WO2014018070 A1, 2014.
Fischer PHH, McDowell CA. The infrared absorption spectra of urea-hydrocarbon adduct. Can J Chem. 1960;38:187–93.
Durie RA, Harrison RJ. Effect of urea-adduct formation and physical state on the infrared spectra of n-paraffin hydrocarbons. Spectrochem Acta. 1962;18:1505–14.
Keller WE. Evidence of planar structure of urea. J Chem Phys. 1948;16:1003–4.
Hoffmann C, Zhang W, Chen Y. Solid forms of a pyrido-pyrimidinium inner salt WO 2013192035 A1, 2013.
Sowa C, Gold RE, Chiodo T, Vogel R. Co-crystals of cyprodinil and dithianon. WO 2013030777 A1, 2013.
McAdie MG. Thermal decomposition of molecular complexes. Can J Chem. 1963;41:2144–53.
Thakral S, Madan AK. Topological models for prediction of heat of decomposition of urea inclusion compounds containing aliphatic endocytes. J Incl Phenom Macrocycl Chem. 2012;60(1):187–92.
White MA. Origins of thermodynamic stability of urea; alkane inclusion compounds. Can J Chem. 1988;76:1695–8.
Radell J, Connolly JW. Urea complexes of partially fluorinated esters. J Org Chem. 1960;25:1202–6.
Carvalho PHV, Jesus AMD, Prata VM, Bezerra DSS, Romao LPC, Navickiene S. Tropical peat as a versatile material for solid-phase extraction of pesticides from medicinal plant cordial salicifolia. J Braz Chem Soc. 2010;. doi:10.1590/S0103-50532010000400011.
Krohl T. Crystalline modification of fipronil. EP 2083628 A1, 2009.
Brodman BW, Radell J. X-ray powder diffraction patterns of some—alkanone urea inclusion compounds. Seperation Sci. 1967;2:139–42.
Radell J, Brodman BW. Urea inclusion compounds of alkenoic acids and alkyl alkenoates. Can J Chem. 1965;43:304–5.
Radell J, Connolly JW. Determination of relative stability of urea complexes from X-ray powder diffraction data. In: Muller WM, editor. Advances in X-ray analysis. New York: Plenum Press; vol. 4, 1961. pp. 140–150.
Harris KDM, Jonsen P. 2H-NMR investigation of the dynamic behavior of n-hexadecane in its urea inclusion compound. Chem Phys Lett. 1989;154:593–8.
Harris KDM. Solid state NMR. Nucl Mag Reson. 1993;22:230–60.
Acknowledgements
Authors are highly thankful to Insecticides (India) Ltd, Chopanki, Bhiwadi (Rajasthan) for providing gift sample of bifenthrin. Authors are thankful to JCDM College of Pharmacy, Sirsa, India, for providing facilities to conduct DSC studies. Authors thank Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, India, for providing facilities for NMR and XRD studies. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of the interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dhall, M., Madan, A.K. Thermal and other analytical studies on bifenthrin urea co-inclusion complex. J Therm Anal Calorim 127, 1639–1653 (2017). https://doi.org/10.1007/s10973-016-6072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6072-8