Abstract
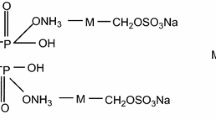
A flame-retardant leather (FR-leather) was prepared from blue wet leather treated with tetrakis hydroxymethyl phosphonium–melamine–pentaerythritol diphosphorate (THPM). An optimized genetic algorithm search method driven by Kissinger method was employed to estimate pyrolysis kinetic mechanism and the thermal stability of FR-leather. It is shown that the thermal decomposition of leather in nitrogen is a three-step kinetic scheme composed of the pyrolysis of triglyceride, multi-complexation collagen and single-complexation collagen, and the activation energies of the corresponding components of FR-leather are, respectively, 84.1, 172.6 and 253.6 kJ mol−1. Accordingly, those of blank leather are 69.3, 161.3 and 105.4 kJ mol−1. Meanwhile, cone calorimeter test demonstrates that when leather was treated with THPM, total heat release and total smoke production are decreased, respectively, 16.2 and 54.5%, and time to ignition increased from 20 s of blank leather to 26 s of FR-leather. The results of limit oxygen index value increased from 26.1% of blank leather to 32.9% of FR-leather, and the results of UL-94 vertical burning tests of FR-leather are significantly decreased, such as flame combustion time and length of carbonization decreased 88% and 72%, respectively. It is shown that the novel THPM material obviously improved the thermal stability and flame-retardant properties of leather fibers; at the same time, it has good smoke suppression effect and is an excellent flame retardant suitable for leather.











Similar content being viewed by others
References
Zhuang LH, Wang GW. Discussion on leather flame retarding treatment and techniques. Leather Sci Eng. 2005;15(3):30.
Huang Z, Li LX, Wang YH, Lin YZ, Chen WY. Performance of flame retardants on leather. J Soc Leather Technol Chem. 2005;89(6):225–31.
Mohamed OA, Abdel-Mohdy FA. Preparation of flame-retardant leather pretreated with pyrovatex CP. J Appl Polym Sci. 2006;99(5):2039–43.
Ling HJ, Yang JW, Xiang L, Li FY, Luo ML, Li LX. The synthesis and application of a high performance amino resin nanocomposite as leather flame retardant. J Soc Leather Technol Chem. 2012;96(1):5–10.
Sanchez-Olivares G, Sanchez-Solis A, Calderas F, Medina-Torres L, Manero O, Di Blasio A, Alongi J. Sodium montmorillonite effect on the morphology, thermal, flame retardant and mechanical properties of semi-finished leather. Appl Clay Sci. 2014;102:254–60.
Jiang YP, Li JX, Li B, Liu HY, Li ZJ, Li LX. Study on a novel multifunctional nanocomposite as flame retardant of leather. Polym Degrad Stab. 2015;115:110–6.
Yang LT, Li Y, Wu YJ, Deng LL, Liu W, Ma CP, Li LX. Thermal degradation kinetics of leather fibers treated with fire-retardant melamine resin. J Therm Anal Calorim. 2015;123(1):413–20.
Li B, Li JX, Li LX, Jiang YP, Li ZJ. Synthesis and application of a novel functional material as leather flame retardant. J Am Leather Chem As. 2014;109(7):239–45.
Cui HW, Jiu JT, Sugahara T, Nagao S, Suganuma K, Uchida H, Schroder KA. Using the Friedman method to study the thermal degradation kinetics of photonically cured electrically conductive adhesives. J Therm Anal Calorim. 2015;119:425–33.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Vincent BJ, Natarajan B. Kinetics of thermal degradation of water borne polyurethane dispersion containing polycaprolactone with either isophorone diisocyanate or metatetramethyl xylene diisocyanate. J Therm Anal Calorim. 2015;119:1373–9.
Ren YL, Cheng BW, Jiang AB, Lu YC, Xu L. Thermal degradation kinetics of poly(O, O-diethyl-O-allylthiophosphate-co-acrylonitrile) in nitrogen. J Appl Polym Sci. 2010;115(6):3705–9.
Flynn JH, Wall LA. General treatment of thermogravimetry of polymers. J Res NBS A Phys Ch. 1966;70:487–9.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Li KY, Huang XY, Fleischmann C, Rein G, Ji J. Pyrolysis of medium-density fiberboard: optimized search for kinetics scheme and parameters via a genetic algorithm driven by Kissinger’s method. Energ Fuel. 2014;28(9):6130–9.
Li KY, Pau DSW, Hou YN, Ji J. Modeling pyrolysis of charring materials: determining kinetic properties and heat of pyrolysis of medium density fiberboard. Ind Eng Chem Res. 2014;53(1):141–9.
Wang YL, Zhao FQ, Ji YP, Yan QL, Yi JH, Xu SY, Luo Y, Lu XM. Synthesis and thermal behaviors of 1,8-dihydroxy-4,5-dinitroanthraquinone barium salt. J Anal Appl Pyrolysis. 2014;105:295–300.
Islam MA, Asif M, Hameed BH. Pyrolysis kinetics of raw and hydrothermally carbonized Karanj (Pongamia pinnata) fruit hulls via thermogravimetric analysis. Bioresour Technol. 2015;179:227–33.
Li J, Gong Y, Li R, Yu XH, Chen WY, Li LX. Research and application of the montmorillonite-amino resin nano-composite flame retardant material. China Leather (Chin). 2008;37(7):43–6.
Wu WX, Mei YF, Zhang L, Liu RH, Cai JM. Effective activation energies of lignocellulosic biomass pyrolysis. Energy Fuel. 2014;28(6):3916–23.
Pau DSW, Fleischmann CM, Spearpoint MJ, Li KY. Determination of kinetic properties of polyurethane foam decomposition for pyrolysis modelling. J Fire Sci. 2013;31(4):356–84.
Liao LL, Chen WY, Shan ZH, Dan WH. Tanning chemistry and technology (in Chinese). Beijing: Science Press; 2005.
Foster JA. Evolutionary computation. Nat Rev Genet. 2001;2(6):428–36.
Lautenberger C, Rein G, Fernandez-Pello C. The application of a genetic algorithm to estimate material properties for fire modeling from bench-scale fire test data. Fire Saf J. 2006;41(3):204–14.
Saha B, Reddy PK, Ghoshal AK. Hybrid genetic algorithm to find the best model and the globally optimized overall kinetics parameters for thermal decomposition of plastics. Chem Eng J. 2008;138:20–9.
Gao M, Wu WH, Liu S, Wang YX, Shen TF. Thermal degradation and flame retardancy of rigid polyurethane foams containing a novel intumescent flame retardant. J Therm Anal Calorim. 2014;117:1419–25.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Item NO. 21176160). The authors deeply appreciate Dr. Kaiyuan Li from the University of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, W., Li, J., Liu, F. et al. Study on the thermal decomposition kinetics and flammability performance of a flame-retardant leather. J Therm Anal Calorim 128, 1107–1116 (2017). https://doi.org/10.1007/s10973-016-5974-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5974-9