Abstract
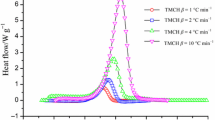
Disasters caused by organic peroxides, such as di(2,4-dichlorobenzoyl) peroxide (DCBP), are mainly attributed to the presence of unstable oxygen atom bonds. In this study, DCBP was investigated through differential scanning calorimetry and thermal activity monitor III, which yielded thermokinetic data to delve into the pure decomposition characteristics of DCBP undergoing chemical reactions. In addition, the thermokinetic data were used to determine the thermal safety parameters through simulation using a best-fit approach based on an appropriately chosen kinetic model and thermal safety software. We found that DCBP decomposes more satisfactorily by an autocatalytic reaction at low temperatures. The apparent activation energy determined through various approaches, such as Kissinger and Ozawa methods, and thermal safety software simulation were studied. Although DCBP decomposition deposits with dioxins, which require major decontamination measures, DCBP is used to produce silicone products globally. The present study establishes threshold thermokinetic parameters for packing and handling thermally sensitive organic peroxides; these thresholds help predict unwarranted runaway reactions, which entail enormous pressure rise and release of toxic by-products to the environment.











Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor of reaction (s−1 M1−n)
- E a :
-
Apparent activation energy (kJ mol−1)
- ΔH d :
-
Heat of decomposition (J g−1)
- k 0 :
-
Pre-exponential factor (s−1)
- k iso :
-
Reaction rate constant under isothermal conditions (h)
- n :
-
Reaction order (dimensionless)
- n i :
-
Reaction order of ith stage (dimensionless)
- Q :
-
Heat production (kJ kg−1)
- R :
-
Universal gas constant (8.314 J mol−1 K−1)
- T :
-
Absolute temperature (K)
- T max :
-
Maximum temperature of reaction (°C or K)
- TMR iso :
-
Time to maximum rate under isothermal conditions (h)
- T o :
-
Apparent exothermic onset temperature of reaction (°C)
- W p :
-
Highest heat flow at time t (w g−1)
- z :
-
Autocatalytic constant (dimensionless)
- α :
-
Degree of conversion (dimensionless)
- β :
-
Heating rate (°C min−1)
References
Swern D, editor. Organic peroxides, vol. 1. Wiely: New York; 1970. p. 82.
Sanders KJ, editor. Organic polymer chemistry. New York: Springer; 1973. p. 347–8.
Dorn M. Environmental aspects of initiators for plastic manufacture and processing. In: Barcelo D, Kostianoy AG, editors. The handbook of environmental chemistry, vol. 12. Heidelberg: Springer; 2010. p. 147–62.
Bhattacharya A, Rawlins JW, Ray P, editors. Polymer grafting and crosslinking. New Jersey: Wiley; 2009. p. 225.
A. Colas, R. Malczewski, K. Ulman. Silicone tubing for pharmaceutical processing. Dow Corning; 2004. www.dowcorning.com.cn.
Park ES. Mechanical properties and antibacterial activity of peroxide-cured silicone rubber foams. J Appl Polym Sci. 2008;110:1723–9.
Liu SH, Hou HY, Chen JW, Weng SY, Lin YC, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;566:226–32.
Chen JR, Wu SH, Lin SY, Hou HY, Shu CM. Utilization of microcalorimetry for an assessment of the potential for a runaway decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2008;93(1):127–33.
Lee MH, Chen JR, Shiue GY, Lin YF, Shu CM. Simulation approach to benzoyl peroxide decomposition kinetics by thermal calorimetric technique. J Taiwan Inst Chem E. 2014;45:115–20.
Chervin S, Bodman GT. Phenomenon of autocatalysis in decomposition of energetic chemicals. Thermochim Acta. 2013;392–393:371–83.
Baquery G, Moine L, Babot O, Degueil M, Maillard B. Model study of crosslinking of polydimethylsiloxanes by peroxides. Polymer. 2005;46:6283–92.
NFPA 432, Code for the storage of the organic peroxide formulations ed. National Fire Protection Association, Quincy; 2002. http://www.nfpa.org.
Reefer container on ship sets off smoke alarm and was found to have over pressurized, UK Chemical Reaction Hazards Forum. http://www.crhf.org.uk.
Fisheick T. An uncontrolled chemical decomposition. Loss Prev Bull. 2004;177:8–9.
Kossoy A, editor. Guidance for advanced simulation-based approach to chemical reactivity evaluation and reaction hazard assessment. Saint Petersburg: ChemInform Saint Petersburg Ltd. (CISP); 2008.
Product data sheet, Aceox® Chemical Corp., 2016. www.acechem.com.tw.
Gabbot P. Principles and applications of thermal analysis. Ames: Blackwell Publishing Ltd.; 2008. p. 1–49.
STARe Software with Solaris Operating System. Operating instructions. Stockholm: Mettler Toledo; 2015.
Product Information, TAM III Thermostat. 2016. www.thermometric.com.
Wei JM, You ML, Chu YC, Shu CM. Evaluation of thermal hazard for lauroyl peroxide by VSP2 and TAM III. Thermochim Acta. 2012;109:1237–43.
Liu SH, Hou HY, Shu CM. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim Acta. 2015;605:68–76.
Liu SH, Hsu CM, Hou HY. Applications of thermal hazard analyses on process safety assessments. J Loss Prev Process Ind. 2015;33:59–69.
Bou-Diab L, Fierz H. Autocatalytic decomposition reactions, hazards and detection. J Hazard Mater. 2002;93:137–46.
Llopiz J, Romero MM, Jerez A, Laureiro Y. Generalization of the Kissinger equation for several kinetic models. Thermochim Acta. 1995;253:205–11.
Blaine RL, Kissinger HE. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6.
Park BD, Jeong HW. Cure kinetics of melamine-formaldehyde resin/clay/cellulose nanocomposites. J Ind Eng Chem. 2010;16:375–9.
Elsabee MZ, Ibrahim SG. Polymerization of dialkyl fumarates by differential scanning calorimetry. Polym Test. 2001;20:173–8.
User’s Guide for ForK Suite. Saint Petersburg: ChemInform Saint Petersburg Ltd. (CISP); 2016.
Lin SY, Shu CM, Tsai YT, Chen WC. Thermal decomposition on Aceox BTBPC mixed with hydrochloric acid. J Therm Anal Calorim. 2015;122:1177–89.
Das M, Shu CM. A green approach towards adoption of chemical reaction model on 2,5-dimethyl-2,5-di-(tert-butylperoxy)hexane decomposition by differential isoconversional kinetic analysis. J Hazard Mater. 2016;301:222–32.
Lin SY, Chen KY, Shu CM. Calorimetric evaluation of polymerization thermokinetics of styrene, α-methylstyrene and trans-ß-methylstyrene. J Hazard Mater. 2009;161:330–5.
Acknowledgements
We express our appreciation to the members at Process Safety and Disaster Prevention Laboratory for the experimental support and research assistance. We are grateful to Dr. Kuang-Hua Hsueh for his expert opinions. We also appreciate Ms. Tzu-Hao Lin and Mr. Shih-Yu Huang for experimental assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, MH., You, ML., Laiwang, B. et al. Isothermal and non-isothermal calorimetric techniques combined with a simulation approach for studying the decomposition characteristics of di(2,4-dichlorobenzoyl) peroxide. J Therm Anal Calorim 127, 1099–1106 (2017). https://doi.org/10.1007/s10973-016-5973-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5973-x