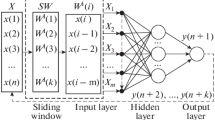
Abstract
The method for evaluation of the results of thermal analysis (TG–DSC) measurements under non-isothermal conditions using neural networks has been described. A requirement of obtaining high-quality neural model was adopted as the criterion. Decomposition of ammonium metavanadate (NH4VO3) in dry air was studied. Empirically determined sets of the results of TG, DTG and DSC measurements and a subset (TG) 3 determined for the third stage of the process were examined. The independent (input) variables were the sample heating rate and the temperature, and the dependent (output) variable was one of the above functions. For the considered data sets, generalized neural networks have been selected. These networks were evaluated based on the criterions taken in the theory of neural networks. High statistical evaluation of the network was received, i.e.,: average error for the set of TG was 0.0397 %, the correlation 0.999957; average error for a subset of (TG) 3 0.118 %, the correlation 0.99995; average error for a set of DTG 0.183 %, the correlation 0.97709; and average error for a set of DSC 0.005 %, the correlation 0.99746. This means, that the sample heating rate and temperature are sufficient to describe the tested functions, the independent and dependent variables were determined with good accuracy, and the sets were numerous sufficiently.







Similar content being viewed by others
Abbreviations
- DSC:
-
Differential scanning calorimetry (mW)
- DSC :
-
Set of values DSC function of all series of measurements
- DTG:
-
Thermogravimetric derivative (% min−1, % K−1)
- DTG :
-
Set of values DTG function of all series of measurements
- TG:
-
Thermogravimetry (mg mg−1)
- TG :
-
Set of values TG function of all series of measurements
- (TG) 3 :
-
Subset of values TG function of all series of measurements relating to the III stage
- T :
-
Temperature (K)
- β :
-
Heating rate (K min−1)
- XRD:
-
X-ray diffraction
- ICDD:
-
International Centre for Diffraction Data
- ANN:
-
Artificial neural network
- SNN:
-
Statistica Neural Network
- MLP:
-
Multilayer perceptron
- RBF:
-
Radial basis function
- GRNN:
-
Generalized neural network
- T r :
-
Training set
- V e :
-
Validation subset
- T e :
-
Testing subset
- IPS:
-
Intelligent problem solver
- Data S.D.:
-
Standard deviation
- Abs. Mean:
-
Mean value of the modules of errors
- Error S.D.:
-
Standard deviation of errors
- S.D. Ratio:
-
Criterion determined as the ratio of Error S.D/S.D. Data
References
Biedunkiewicz A. Aspects of manufacturing of ceramic nanomaterials of TiC/C, TiC, TiC–SiC–C and Ti(C, N)–Si(C, N)–Si3N4 type by the sol–gel method. Szczecin: West Pomeranian University of Technology; 2009 (in Polish).
Krawczyk M. Nanometric carbides in the Ti–Mo–Si system obtained by sol–gel method, Doctoral dissertation. Szczecin: West Pomeranian University of Technology; 2013 (in Polish).
Biedunkiewicz A, Figiel P, Krawczyk M, Gabriel-Polrolniczak U. Simultaneous synthesis of molybdenum carbides and titanium carbides by sol–gel method. J Therm Anal Calorim. 2013;113:253–8.
Biedunkiewicz A. Methodology of thermal research in materials engineering. In: Czerwinski F, editor. Heat treatment—conventional and novel applications, Rijeka, HR; 2012. p. 135.
Strzelczak A. Analysis of oxidation processes of selected ceramic nanocomposites in dry air, Doctoral dissertation. Szczecin: Szczecin University of Technology; 2008 (in Polish).
Biedunkiewicz A, Strzelczak A, Możdżeń G, Lelątko J. Non-isothermal oxidation of ceramic nanocomposites using the example of Ti–Si–C–N powder: kinetic analysis method. Acta Mater. 2008;56:3132–45.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Straszko J, Biedunkiewicz A, Strzelczak A. Application of artificial neural networks in oxidation kinetic analysis of nanocomposites. Pol J Chem Technol. 2008;10:21–8.
Mc Culloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;9:115–33.
Hopfield J. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA. 1982;79:2554–8.
Osowski S. Neural networks in terms of algorithmic. Warszawa: WNT; 1996 (in Polish).
Tadusiewicz R. Neural networks. Warszawa: Akademicka Oficyna Wydawnicza; 1993 (in Polish).
Statistica—Program description Statsoft. http://statsoft.pl. (in Polish).
Statistica Neural Networks—Introduction for neural networks. http://statsoft.pl. (in Polish).
Statistica Neural Networks—Problem guide. http://statsoft.pl. (in Polish).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krawczyk, M., Figiel, P. Evaluation of thermogravimetric measurements using neural networks. J Therm Anal Calorim 126, 585–592 (2016). https://doi.org/10.1007/s10973-016-5539-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5539-y