Abstract
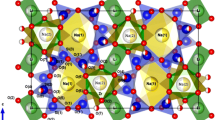
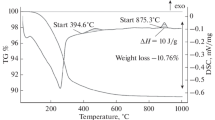
This paper was devoted to explanation of the phase transitions nature in Ca12Al14O33±δ which were studied by a combination of methods: simultaneous thermal analysis, high-temperature X-ray diffraction analysis, Raman spectroscopy. One of the phase transitions is assumed to be connected with peroxide ions appearance/disappearance in mayenite at 922 K. The peroxide ions appearance reduces the system’s energy. Peroxide ions are responsible for the interaction of mayenite with water giving hydroxyl ions. Another phase transition at 1268 K is caused by changing in stability of electronic defects in mayenite. This paper enlightens correlations between the electronic defects state, the peroxide ions existence and chemical properties of Ca12Al14O33±δ .









Similar content being viewed by others
References
Kofstad PK. Nonstoichiometry, diffusion and electrical conductivity in binary metal oxides (science and technology of materials). New York: Wiley; 1972.
West AR. Solid state chemistry and it’s applications V1. New York: Wiley; 1984.
Artacho E, Yndurain F, Pajot B, Ramırez R, Herrero CP, Khirunenko LI, Itoh KM, Haller EE. Interstitial oxygen in germanium and silicon. Phys Rev B. 1997;56(7):3820–33.
Imlach JA, Glasser LSD, Glasser FP. Excess oxygen and the stability of 12CaO·7Al2O3. Cement Concrete Res. 1971;1:57–61.
Christensen AN. Neutron powder diffraction profile refinement studies on Ca11.3Al14O32.3 and CaClO(D0.88H 0.12). Acta Chem Scand Ser A Phys Inorg Chem. 1987;41(2):110–2.
Boysen H, Lerch M, Stys A, Senyshyn A. Structure and oxygen mobility in mayenite (Ca12Al14O33): a high-temperature neutron powder diffraction study. Acta Cryst. 2007;B63:675–8.
Janek J, Lee D-K. Defect chemistry of the mixed conducting cage compound Ca12Al14O33. J Korean Ceram Soc. 2010;47(2):99–105.
Hayashi K, Hirano M, Matsuishi S, Hosono H. Microporous crystal 12CaO·7Al2O3 encaging abundant O− radicals. J Am Chem Soc. 2002;124(5):738–9.
Jeevaratnam J, Glasser FP, Glasser LSD. Anion substitution and structure of 12CaO·7Al2O3. J Am Ceram Soc. 1964;47(2):105–6.
Lacerda M, Irvine JTS, Glasser FP, West AR. High oxide ion conductivity in Ca12Al14O33. Nature. 1988;332(7):525–6.
Hayashi K, Matsuishi S, Kamiya T, Hirano M, Hosono H. Light-induced conversion of an insulating refractory oxide into a persistent electronic conductor. Nature. 2002;419(3):462–4.
Nurse RW, Welch JH, Majumdar AJ. The 12CaO·7Al2O3 phase in the CaO–Al2O3 system. Trans Br Ceram Soc. 1965;64:323–332.
Hayashi K, Hirano M, Hosono H. Thermodynamics and kinetics of hydroxide ion formation in 12CaO·7Al2O3. J Phys Chem B. 2005;109:11900(7).
Tolkacheva AS, Shkerin SN, Korzun IV, Plaksin SV, Khrustov VR, Ordinartsev DP. Phase transition in mayenite Ca12Al14O33. Russ J Inorg Chem. 2012;57(7):1014–5 (in Russian P. 1089–5).
Jeevaratnam J, Glasser LSD, Glasser FP. Structure of calcium aluminate 12CaO·7Al2O3. Nature. 1962;194(4830):764–5.
Yoon SG, Kim SW, Hirano M, Yoon DH, Hosono H. Pore-free 12CaO·7Al2O3 single-crystal growth by melt state control using the floating zone method. Crystal Growth Des. 2008;8(4):1271–5.
Yang S, Kondo JN, Hayashi K, Hirano M, Domen K, Hosono H. Formation and desorption of oxygen species in nanoporous crystal 12CaO·7Al2O3. Chem Mater. 2004;16:104–7.
Hosono H. Functioning of traditional ceramics 12CaO·7Al2O3 utilizing built-in nano-porous structure. Sci Technol Adv Mater. 2004;5:409–16.
Sushko PV, Shluger AL, Hirano M, Hosono H. From insulator to electride: a theoretical model of nanoporous oxide 12CaO·7Al2O3. J Am Chem Soc. 2007;129:942–51.
Matsuishi S, Kim SW, Kamiya T, Hirano M, Hosono H. Localized and delocalized electrons in room-temperature stable electride [Ca24Al28O64]4+(O2−)2−x(e−)2x: analysis of optical reflectance spectra. J Phys Chem C. 2008;112(12):4753–8.
Kim SW, Matsuishi S, Miyakawa M, Hayashi K, Hirano M, Hosono H. Fabrication of room temperature-stable 12CaO·7Al2O3 electride: a review. J Mater Sci Mater Electron. 2007;18:S5–14.
Kamiya T, Hosono H. Built-in quantium dots in nano-porous crystal 12CaO·7Al2O3: simplified views for electronic structure and carrier transport. Jpn J Appl Phys. 2005;44(1B):774–9.
McLeod JA, Buling A, Kurmaev EZ, Sushko PV, Neumann M, Finkelstein LD, Kim SW, Hosono H, Moewes A. Experimental evidence of cage conduction bands in superconducting cement 12CaO·7Al2O3. Phys Rev B. 2012. doi:10.1103/PhysRevB.85.045204.
Matsuishi S, Hayashi K, Hirano M, Tanaka I, Hosono H. Superoxide ion encaged in nanoporous crystal 12CaO·7Al2O3 studied by continuous wave and pulsed electron paramagnetic resonance. J Phys Chem B. 2004;108:18557–68.
Hayashi K, Ueda N, Hirano M, Hosono H. Effect of stability and diffusivity of extra-framework oxygen species on the formation of oxygen radicals in 12CaO·7Al2O3. Solid State Ion. 2004;173(1–4):89–94.
Ruszak M, Witkowski S, Sojka Z. EPR and Raman investigations into anionic redox chemistry of nanoporous 12CaO·7Al2O3 interacting with O2, H2 and N2O. Res Chem Intermed. 2007;33(8–9):689–703.
Kajihara K, Matsuishi S, Hayashi K, Hirano M, Hosono H. Vibrational dynamics and oxygen diffusion in a nanoporous oxide ion conductor 12CaO·7Al2O3 studied by 18O labeling and micro-Raman spectroscopy. J Phys Chem C. 2007;111:14855–7.
Nishioka M, Nanjyo H, Hamakawa S, Kobayashi K, Sato K, Inoue T, Mizukami F, Sadakata M. O− emission from 12CaO·7Al2O3 and MSZ composite and its application for silicon oxidation. Solid State Ion. 2006;177:2235–9.
Tolkacheva AS, Shkerin SN, Plaksin SV, Vovkotrub EG, Bulanin KM, Kochedykov VA, Ordinartsev DP, Gyrdasova OI, Molchanova NG. Synthesis of dense ceramics of single-phase mayenite (Ca12Al14O32)O. Russ J Appl Chem. 2011;84(6):907–11 (original Russian text published in Zhurnal prikladnoi khimii 84(6): 881–7).
Olszak-Humienik M, Jablonski M. Thermal behavior of natural dolomite. J Thermal Anal Calorim. 2015;119(3):2239–48.
Madej D, Szczerba J. Study of the hydration of calcium zirconium aluminate (Ca7ZrAl6O18) blended with reactive alumina by calorimetry, thermogravimetry and other methods. J Thermal Anal Calorim. 2015;121(2):579–88.
Chebotin V., Perfiliev M. Electrochemistry of solid electrolytes. Washington: Technical Information Center, U.S. Department of Energy; 1984.
Lee D-K, Kogel L, Ebbinghaus S, Valov I, Wiemhoefer H-D, Lerch M, Janek J. Defect chemistry of the cage compound, Ca12Al14O33-δ—understanding the route from a solid electrolyte to a semiconductor and electride. Phys Chem Chem Phys. 2009;11:3105–10.
I. I. Vol’nov Peroxide compounds of Alkaline Earth Metals. M.:Nauka, 1983 in Russian/was republished in English I. I. Vol’nov Peroxides, Superoxides, and Ozonides of Alkali and Alkaline Earth Metals. US: Springer, http://www.springer.com/gp/book/9781468482546?wt_mc=ThirdParty.SpringerLink.3.EPR653.About_eBook.
Rankin GA, Wright FE. Ternary system CaO–Al2O3–SiO2. Am J Sci 4th Ser. 1915;39(1):1–79.
Hallstedt B. Assessment of the CaO–Al2O3 system. J Am Ceram Soc. 1990;73(1):15–9.
Büssem W, Eitel A. Die Struktur des Pentacalciumaluminats. Z Krist. 1936;95:175–88.
Welch JH. Chemistry of cements. 2 ed. V. 1. Taylor HFW, editor. London: Academic Press; 2004.
Acknowledgements
The authors are grateful to Chebykin V.V. for the thermodynamic simulations on the HSC5 program, the colleagues from the shared access center “Ural-M” Titova S.G. and Fedorova O.M. for the X-ray high-temperature investigation. A part of the X-ray investigation was performed by Antonov B.D. Also we express our thanks to the shared access center “Composition of compounds” Institute of High-Temperature Electrochemistry Ural Branch of the Russian Academy of Sciences for providing facilities for structural investigations. The research work was financially supported by the Russian Foundation for Basic Research (Grant Nos. 13-08-96020, 14-03-31091) and the Government of Sverdlovsk Region.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shkerin, S.N., Tolkacheva, A.S., Korzun, I.V. et al. Phase transitions in mayenite. J Therm Anal Calorim 124, 1209–1216 (2016). https://doi.org/10.1007/s10973-016-5282-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5282-4