Abstract
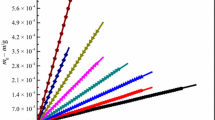
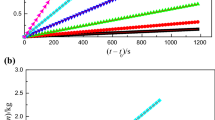
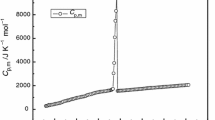
Lactic acid ionic liquid [C4mim][Lact] (1-butyl-3-methylimidazolium lactic acid) was synthesized by the neutralization method. Using the solution–reaction isoperibol calorimeter, the molar enthalpies of solution, Δsol H m, with different molalities were determined in the temperature range from (288.15 to 308.15 ± 0.01) K with an interval of 5 K. In terms of Archer’s method, the standard molar enthalpy of solution for [C4mim][Lact], \( \Delta _{\text{sol}} H_{\text{m}}^{\theta } \), was obtained, and then, the values of apparent relative molar enthalpy, Φ L, and the Pitzer’s parameters \( \beta_{\text{MX}}^{(0)\text{L}} \) and \( \beta_{\text{MX}}^{(1)\text{L}} \) were determined for [C4mim][Lact]. The plot of \( \Delta _{\text{sol}} H_{\text{m}}^{\theta } \) versus (T—298.15) K is a good straight line, and its slope is the standard molar heat capacity of solution, \( \Delta C_{\text{p,m}}^{\theta } = 240\,{\text{J}}\,{\text{K}}^{ - 1} \). According to Glasser’s theory of lattice energy, the hydration enthalpy of cation and anion, (ΔH + + ΔH −) = −471 kJ mol−1, in infinite dilution and the hydration enthalpy of anion, ΔH −([Lact]−) = −257 kJ mol−1, were obtained at 298.15 K. The heat capacity of aqueous [C4mim][Lact], C p(sol), and the apparent molar heat capacity, Φ C p, of various specific molalities were also obtained.



Similar content being viewed by others
References
Murillo-Hernández JA, López-Ramírez S, Domínguez JM, Duran-Valencia C, García-Cruz I, González-Guevara JA. Survey on ionic liquids effect based on metal anions over the thermal stability of heavy oil. J Therm Anal Calorim. 2009;95:173–9.
Pawlak K, Szurkowski J, Skrzypczak A, Bialek-Bylka GE. The thermal deactivation of all-trans and 15-cis beta-carotene-excited states in the ionic liquids without and with methylenoxy group. J Therm Anal Calorim. 2015;120:627–32.
Zhang SJ, Wang JJ, Lu XM, Zhou Q. Structures and interactions of ionic liquids. Heidelberg: Springer; 2014. p. 151–97.
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. J Am Chem Soc. 2005;127:2398–9.
Wagner V, Schulz PS, Wasserscheid P. Asymmetric hydrogenation catalysis via ion-pairing in chiral ionic liquids. J Mol Liq. 2014;192:177–84.
Navarro P, Larriba M, Beigbeder JB. Thermal stability and specific heats of [bpy][BF4] + [bpy][Tf2N] and [bpy][BF4] + [4bmpy][Tf2N] mixed ionic liquid solvents. J Therm Anal Calorim. 2015;119:1235–43.
Schulz PS, Mller N, Bsmann A, Wasserscheid P. Effective chirality transfer in ionic liquids through ion-pairingeffects. Angew Chem. 2007;119:1315–7.
Wu JW, Su P, Huang J, Wang SM, Yang Y. Synthesis of teicoplanin-modified hybrid magnetic mesoporous silica nanoparticles and their application in chiral separation of racemic compounds. J Colloid Interface Sci. 2013;399:107–14.
Baudequin C, Bregeon D, Levillain J, Guillen F, Plaquevent JC, Gaumont AC. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron Asymmetry. 2005;16:3921–45.
Kapnissi-Christodoulou CP, Stavrou IJ, Mavroudi MC. Chiral ionic liquids in chromatographic and electrophoretic separations. J Chromatogr A. 2014;1363:2–10.
Guan W, Xue WF, Li N, Tong J. Enthalpy of solution of amino acid ionic liquid 1-butyl-3-methylimidazolium glycine. J Chem Eng Data. 2008;53:1401–3.
Guan W, Li L, Wang H, Tong J, Yang JZ. study on thermochemical properties of ionic liquids based on transition metal. J Therm Anal Calorim. 2008;94(2):507–10.
Archer DG, Widegren JA, Kirklin DR, Magee JW. Enthalpy of solution of 1-octyl-3-methylimidazolium tetrafluoroborate in water and in aqueous sodium fluoride. J Chem Eng Data. 2005;50:1484–91.
Wilkes JS, Levisky JA, Wilson RA, Hussey CL. Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry. Inorg Chem. 1982;21:1263–8.
Di YY, Qu SS, Liu Y, Wen DC, Tang H, Li LW. A thermochemical study of the solid-state coordination reactions of two α-amino acids with copper(II) acetate. J Thermochim Acta. 2002;387:115–9.
Liu JG, Xue WF, Qin Y, Yan CW. Enthalpy of solution for anhydrous VOSO4 and estimated enthalpy of reaction for formation of the ion pair [VOSO4]0. J Chem Eng Data. 2009;54:1938–41.
Ji M, Liu MY, Gao SL, Shi QZ. A new microcalorimeter for measuring thermal effects. Instrum Sci Technol. 2001;29:53–7.
Montgomery RL, Melaugh RA, Lau CC, Meier GH, Chan HH, Rossini FD. Determination of the energy equivalent of a water solution calorimeter with a standard substance. J Chem Thermodyn. 1977;9:915–36.
Rychly R, Pekarek V. The use of potassium chloride and tris(hydroxymethyl)aminomethane as standard substances for solution calorimetry. J Chem Thermodyn. 1977;9:391–6.
Pitzer KS. In activity coefficients in electrolyte solution, Chapter 3. Boca Raton, FL: CRC Press; 1991.
Glasser L. Lattice and phase transition thermodynamics of ionic liquids. Thermochim Acta. 2004;421:87–93.
Glasser L, Jenkins HDB. Volume-based thermodynamics: a prescription for its application and usage in approximation and prediction of thermodynamic data. J Chem Eng Data. 2011;56:874–80.
Gutowski KE, Rogers RD, Dixon DA. Accurate thermochemical properties for energetic materials applications. II. Heats of formation of imidazolium-, 1,2,4-triazolium-, and tetrazolium-based energetic salts from isodesmic and lattice energy calculations. J Phys Chem B. 2007;111:4788–800.
Fang DW, Tong J, Guan W, Wang H, Yang JZ. Prediction of the thermodynamic properties of 1-alkyl-3-methylimidazolium lactate ionic liquids [Cnmim][Lact](n = 2, 3, 4, 5, and 6) by parachor. Sci Sin Chim. 2010;40(9):1339–47.
Guan W, Yang JZ, Li L, Wang H, Zhang QG. Thermochemical properties of aqueous solution containing ionic liquids. 1. The heat of reaction mixed 1-methyl-3-butylimidazolium chloride with InCl3. Fluid Phase Equilib. 2006;239:161–75.
Liang XY, Zhang X, Wang SG, Li W, Rong H. A study on molar enthalpy of solution in amino acid tetrafluoroborate ionic liquids. J Beijing Inst Petrochem Technol. 2011;19:50–3.
Paulechka YU, Kabo AG, Blokhin AV, Kabo GJ, Shevelyova MP. Heat capacity of ionic liquids: experimental determination and correlations with molar volume. J Chem Eng Data. 2010;55:2719–24.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (21173107) and Liaoning Excellent Talents in University (2015025).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, L., Bu, XX., Fan, BH. et al. Study on thermodynamic property for ionic liquid [C4mim][Lact](1-butyl-3-methylimidazolium lactic acid). J Therm Anal Calorim 123, 1619–1625 (2016). https://doi.org/10.1007/s10973-015-5051-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5051-9