Abstract
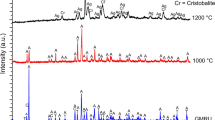
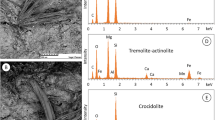
This paper reports a systematic and comparative study of the thermal behaviour of fibres of social, health, economic and industrial relevance using thermogravimetric and differential scanning calorimetry (TG/DSC). The mineral fibres selected for the study are: three chrysotile samples, crocidolite, tremolite asbestos, amosite, anthophyllite asbestos and asbestiform erionite. Powder X-ray diffraction and scanning electron microscopy were used for the characterization of the mineral fibres before and after heating at 1000 or 1100 °C to identify the products of the thermal decomposition at a microscopic and structural scale and characterize their thermal behaviour. TG/DSC data allowed the determination of the structural water content and temperature stability. Furthermore, thermal analysis provided a sensitive and reliable technique for the detection of small quantities of different mineral phases occurring as impurities. After thermal treatment, fibrous samples were completely transformed into various iron oxide, cristobalite and other silicate phases which preserved the original overall fibrous morphology (as pseudomorphosis). Only crocidolite at 1100 °C was partially melted, and an amorphous surface was observed.

















Similar content being viewed by others
References
Guthrie GD, Mossman BT. Merging the geological and biological science an integrated approach to mineral induced pulmonary disease. In: Guthrie GD, Mossman BT, editors. Health effects of mineral dusts, Vol. 28, Reviews in mineralogy and geochemistry. Chelsea: Mineralogical Soc. America Geochemical Soc; 1993. p. 1–5.
Francine B, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol. 2013;14:576–8.
Whittaker EJW. The structure of chrysotile. V. Diffuse reflections and fibre texture. Acta Crystallogr. 1957;10:149–56.
Yada K. Study of microstructure of chrysotile asbestos by high-resolution electron microscopy. Acta Crystallogr. 1971;A 27:659–64.
Smith JV, Bennett JM. Enumeration of 4-connected 3-dimensional nets and classification of framework silicates; the infinite set of ABC-6 nets; the Archimedean and sigma-related nets. Am Mineral. 1981;66:777–88.
Gualtieri G, Artioli E, Passaglia S, Bigi A, Viani JCH. Crystal structure-crystal chemistry relationships in the zeolites erionite and offretite. Am Mineral. 1998;83:590–606.
Gunter ME, Belluso E, Mottana A. Amphiboles: environmental and health concerns. In: Rosso JJ, editor. Reviews in mineralogy and geochemistry. Chantilly: Mineralogical Society of America Geochemical Society; 2007. p. 453–516.
Kamp DW. Asbestos-induced lung diseases: an update. Transl Res. 2009;153:143–52.
Bertino P, Marconi A, Palumbo L, Bruni BM, Barbone D, Germano S, Dogan AU, Tassi GF, Porta C, Mutti L, Gaudino G. Erionite and asbestos differently cause transformation of human mesothelial cells. Int J Cancer. 2007;121:12–20.
Plescia P, Gizzi D, Benedetti S, Camilucci L, Fanizza C, De Simone P, Paglietti F. Mechanochemical treatment to recycling asbestos-containing waste. Waste Manage. 2003;23:209–18.
Favero-Longo SE, Castelli D, Fubini B, Piervittori R. Lichens on asbestos-cement roofs: bioweathering and biocovering effects. J Hazard Mater. 2009;162:1300–8.
Anastasiadou K, Axiotis D, Gidarakos E. Hydrothermal conversion of chrysotile asbestos using near supercritical conditions. J Hazard Mater. 2010;179:926–32.
Leonelli C, Veronesi P, Boccaccini DN, Rivasi MR, Barbieri L, Andreola F, Lancellotti I, Rabitti D, Pellacani GC. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J Hazard Mater. 2006;135:149–55.
Boccaccini DN, Leonelli C, Rivasi MR, Romagnoli M, Veronesi P, Pellacani GC, Boccaccini AR. Recycling of microwave inertised asbestos containing waste in refractory materials. J Eur Ceram Soc. 2007;27:1855–8.
Candela PA, Crummett CD, Earnest DJ, Frank MR, Wylie AG. Low-pressure decomposition of chrysotile as a function of time and temperature. Am Mineral. 2007;92:1704–13.
Gualtieri AF, Cavenati C, Zanatto I, Meloni M, Elmi G, Lassinantti Gualtieri M. The transformation sequence of cement–asbestos slates up to 1200 °C and safe recycling of the reaction product in stoneware tile mixtures. J Hazard Mater. 2008;152:563–70.
Zaremba T, Peszko M. Investigation of the thermal modification of asbestos wastes for potential use in ceramic formulation. J Therm Anal Calorim. 2008;92:873–7.
Dellisanti F, Rossi PL, Valdre G. Remediation of asbestos containing materials by Joule heating vitrification performed in a pre-pilot apparatus. Int J Miner Process. 2009;91:61–7.
Yvon Y, Sharrock P. Characterization of thermochemical inactivation of asbestos containing wastes and recycling the mineral residues in cement products. Waste Biomass Valor. 2011;2:169–81.
Kusiorowski R, Zaremba T, Piotrowski J, Gerle A. Thermal decomposition of asbestos-containing materials. J Therm Anal Calorim. 2013;113:179–88.
Cattaneo A, Gualtieri AF, Artioli G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys Chem Miner. 2003;30:177–83.
Zaremba T, Krząkała A, Piotrowski J, Garczorz D. Study on the thermal decomposition of chrysotile asbestos. J Therm Anal Calorim. 2010;101:479–85.
Zulumyan N, Mirgorodski A, Isahakyan A, Beglaryan H. The mechanism of decomposition of serpentines from peridotites on heating. J Therm Anal Calorim. 2014;115:1003–12.
Hodgson AA, Freeman AG, Taylor HFW. The thermal decomposition of crocidolite from Koegas, South Africa. Mineral Mag. 1965;35:5–30.
Rouxhet PG, Gillard JL, Fripiat JJ. Thermal decomposition of amosite, crocidolite, and biotite. Mineral Mag. 1972;38:583–92.
Kohyama N, Shinohama Y, Suzuki Y. Mineral phases and some re-examined characteristics of the international union against cancer standard asbestos samples. Am J Ind Med. 1996;30:515–28.
Jeyaratnam M, West NG. A study of heat-degraded chrysotile, amosite and crocidolite by X-ray diffraction. Ann Occup Hyg. 1994;38:137–48.
Gualtieri AF, Levy D, Belluso E, Dapiaggi M. Kinetics of the decomposition of crocidolite asbestos: a preliminary real-time X-ray powder diffraction study. Miner Sci Forum. 2004;443–444:291–4.
Kusiorowski R, Zaremba T, Gerle A, Piotrowski J, Simka W, Adamek J. Study on the thermal decomposition of crocidolite asbestos. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4421-7.
Hodgson AA, Freeman AG, Taylor HFW. The thermal decomposition of amosite. Mineral Mag. 1965;35:445–63.
Freeman AG. The dehydroxylation behavior of amphibole. Mineral Mag. 1966;35:953–7.
Kusiorowski R, Zaremba T, Piotrowski J, Adamek J. Thermal decomposition of different types of asbestos. J Therm Anal Calorim. 2012;109:693–704.
Papke KG. Erionite and associated zeolites in Nevada. Nev Bur Mines Geol Bull. 1972;79:1–31.
Pugnaloni A, Giantomassi F, Lucarini G, Capella S, Bloise A, Di Primio R, Belluso E. Cytotoxicity induced by exposure to natural and synthetic tremolite asbestos: an in vitro pilot study. Acta Histochem. 2013;115:100–12.
Duncan KE, Cook PM, Gavett SH, Dailey LA, Mahoney RK, Ghio AJ, Roggli VL, Devlin RB. In vitro determinants of asbestos fiber toxicity: effect on the relative toxicity of Libby amphibole in primary human airway epithelial cells. Part Fibre Toxicol. 2014;11:1–14.
Pollastri S, Gualtieri AF, Lassinantti Gualtieri M, Hanuskova M, Cavallo A, Gaudino G. The zeta potential of mineral fibres. J Hazard Mater. 2014;276:469–79.
Frost RL, Erickson KL. Thermal decomposition of synthetic hydrotalcites reevesite and pyroaurite. J Therm Anal Calorim. 2004;76:217–25.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Gallagher PK, Warne SSTJ. Thermomagnetometry and thermal decomposition of siderite. Thermochim Acta. 1981;43:253–67.
Viti C. Serpentine minerals discrimination by thermal analysis. Am Mineral. 2010;95:631–8.
Villieras F, Yvon J, Cases JM, De Donato P, Lhote F, Baeza R. Development of microporosity in clinochlore upon heating. Clay Clay Miner. 1994;42:679–88.
Catalano M, Belluso E, Miriello D, Barrese E, Bloise A. Synthesis of Zn-doped talc in hydrothermal atmosphere. Cryst Res Technol. 2014;49:283–9.
Ball MC, Taylor HFW. The dehydration of chrysotile in air and under hydrothermal conditions. Mineral Mag. 1963;33:467–82.
Brindley GW, Hayami R. Mechanism of formation of forsterite and enstatite from serpentine. Mineral Mag. 1965;35:189–95.
Martin CJ. The thermal decomposition of chrysotile. Mineral Mag. 1977;41:453–9.
MacKenzie KJD, Meinhold RH. Thermal reactions of chrysotile revisited: a 29 Si and 25 Mg MAS NMR study. Am Mineral. 1994;79:43–50.
Bloise A, Belluso E, Barrese E, Miriello D, Apollaro C. Synthesis of Fe-doped chrysotile and characterization of the resulting chrysotile fibers. Cryst Res Technol. 2009;44:590–6.
Bloise A, Belluso E, Fornero E, Rinaudo C, Barrese E, Capella S. Influence of synthesis conditions on growth of Ni-doped chrysotile. Micropor Mesopor Mat. 2010;132:239–45.
Bloise A, Critelli T, Catalano M, Apollaro C, Miriello D, Croce A, Barrese E, Liberi F, Piluso E, Rinaudo C, Belluso E. Asbestos and other fibrous minerals contained in the serpentinites of the Gimigliano-Mount Reventino Unit (Calabria, S-Italy). Environ Earth Sci. 2014;71:3773–86.
Kulp JL, Trites AF. Differential thermal analysis of natural hydrous ferric oxides. Am Mineral. 1951;36:23–44.
Taufiq-Yap YH, Nur-Faizal AR, Sivasangar S, Hussein MZ, Aishah A. Modification of Malaysian dolomite using mechanochemical treatment via different media for oil palm fronds gasification. Int J Energy Res. 2014;38:1008–15.
Viti C, Giacobbe C, Gualtieri AF. Quantitative determination in massive serpentinites using DTA: implications for asbestos determination. Am Mineral. 2011;96:1003–11.
Cattaneo A, Somigliana A, Gemmi M, Bernabeo F, Savoca D, Cavallo DM, Bertazzi PA. Airborne concentrations of chrysotile asbestos in serpentine quarries and stone processing facilities in valmalenco, Italy. Ann Occup Hyg. 2012;56:1–13.
Lesci IG, Balducci G, Pierini F, Soavi F, Roveri N. Surface features and thermal stability of mesoporous Fe doped geoinspired synthetic chrysotile nanotubes. Micropor Mesopor Mat. 2014;197:8–16.
Lafay R, Montes-Hernandez G, Janots E, Auzende AL, Chiriac R, Lemarchand D, Toche F. Influence of trace elements on the textural properties of synthetic chrysotile: complementary insights from macroscopic and nanoscopic measurements. Micropor Mesopor Mat. 2014;183:81–90.
Wypych F, Schreiner WH, Mattoso N, Mosca DH, Marangonia R, Bento CAS. Covalent grafting of phenylphosphonate groups onto layered silica derived from in situ-leached chrysotile fibers. J Mater Chem. 2003;13:304–7.
Giacobbe C, Gualtieri AF, Quartieri S, Rinaudo C, Allegrina M, Andreozzi GB. Spectroscopic study of the product of thermal transformation on chrysotile-asbestos containing materials. Eur J Mineral. 2010;22:535–46.
Croce A, Allegrina M, Trivero P, Rinaudo C, Viani A, Pollastri S, Gualtieri AF. The concept of ‘end of waste’ and recycling of hazardous materials: in depth characterization of the product of thermal transformation of cement-asbestos. Mineral Mag. 2014;78:1177–91.
Fujishige M, Kuribara A, Karasawa I, Kojima A. Low-temperature pyrolysis of crocidolite and amosite using calcium salts as a flux. J Ceram Soc Jpn. 2007;115:434–9.
Addison CC, Addison WE, Neal GA, Sftarv JH. Amphiboles Part I: the oxidation of crocidolite. J Chem Soc. 1962;278:1468–71.
Brindley GW, Youell RF. Ferrous chamosite and ferric chamosite. Mineral Mag. 1953;30:57–70.
Yagi K. The system acmite-diopside and its bearing on the stability relations of natural pyroxenes of the acmite–hedenbergite–diopside series. Am Mineral. 1966;51:976–1000.
Bowen NL, Schairer JF. The fusion relations of acmite. Am J Sci. 1929;18:365–74.
MacKenzie RC. The differential thermal investigation of clays. London: Mineralogical Society (Clay Minerals Group); 1957.
Jones AA. Charges on the surfaces of two chlorites. Clay Miner. 1981;16:347–59.
Luckewicz W. Differential thermal analysis of chrysotile asbestos in pure talc and talc containing other minerals. J Soc Cosmet Chem. 1975;26:431–7.
Bloise A, Fornero E, Belluso E, Barrese E, Rinaudo C. Synthesis and characterization of tremolite asbestos fibres. Eur J Mineral. 2008;20:1027–33.
Gualtieri AF, Venturelli P. In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. Am Mineral. 1999;84:895–904.
Miriello D, Bloise A, De Francesco A, Crisci GM, Chiaravalloti F, Barca D, La Russa MF, Marasco E. Colour and composition of nodules from the Calabrian clay deposits: a possible raw material for pigments production in Magna Graecia. Period Mineral. 2010;79:59–69.
Moore GSM, Rose HE. The structure of powdered quartz. Nature. 1973;242:187–90.
Brydon JE, Turner RC. The nature of Kenya vermiculite and its aluminum hydroxide complexes. Clay Clay Miner. 1972;20:1–11.
Bagin VI, Gendler TS, Dainyak LG, Kuz’min RN. Mossbauer, thermomagnetic, and x-ray study of cation ordering and high-temperature decomposition in biotite. Clay Clay Miner. 1980;28:188–96.
Ballirano P, Cametti G. Dehydration dynamics and thermal stability of erionite-K: experimental evidence of the “internal ionic exchange” mechanism. Micropor Mesopor Mat. 2012;163:160–8.
Bieniok A, Bornholdt K, Brendel U, Baur WH. Synthesis and crystal structure of zeolite W, resembling the mineral merlinoite. J Mater Chem. 1996;6:271–5.
Skofteland BM, Ellestad OH, Lillerud KP. Potassium merlinoite: crystallization, structural and thermal properties. Micropor Mesopor Mat. 2001;43:61–71.
Ballirano P, Andreozzi GB, Dogan M, Dogan AU. Crystal structure and iron topochemistry of erionite-K from Rome, Oregon, U.S.A. Am Mineral. 2009;94:1262–70.
Gottardi G, Galli E. Natural zeolites. Berlin: Springer; 1985.
Acknowledgements
This research was conducted within the granted Italian National PROGETTO DI UNA UNITÀ DI RICERCA (PRIN) 2010–2011 –prot. 2010MKHT9B 004 “Interazione fra minerali e biosfera: con-seguenze per l’ambiente e la salute umana”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bloise, A., Catalano, M., Barrese, E. et al. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J Therm Anal Calorim 123, 2225–2239 (2016). https://doi.org/10.1007/s10973-015-4939-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4939-8