Abstract
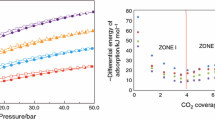
This paper shows the results of the adsorption and reaction of NO on porous solid samples prepared from waste orange peel. Activated carbons prepared from activation with CO2 were impregnated with Ce, Pt, Ni and Fe. These samples were analysed by isotherms of N2 at −196 °C and isotherms of CO2 at 0 °C. The Ce–Pt-activated carbon from orange peel catalysts are the catalysts with the highest reactivity towards NO. Additionally, the differential heats of adsorption were determined. The results show that the NO adsorption capacity of the metal is a function of the amount of metal impregnated and the textural properties. Adsorption microcalorimetry proved to be a very suitable technique for tracking the reaction of NO on catalysts.








Similar content being viewed by others
References
Chmielarz L, Kustrowski P, Dziembaj R, Cool P, Vansant EF. Catalytic performance of various mesoporous silicas modified with copper or iron oxides introduced by different ways in the selective reduction of NO by ammonia. Appl Catal B: Environ. 2006;62(3–4):369–80.
Calleja G, Aguado J, Carrero A, Moreno J. Preparation, characterization and testing of Cr/AlSBA-15 ethylene polymerization catalysts. Appl Catal A: Gen. 2007;316(1):22–31.
Boutros M, Trichard JM, Costa PD. Silver-supported mesoporous SBA-15 as potential catalysts for SCR NO x by ethanol. Appl Catal B: Environ. 2009;91(3–4):640–8.
Brandenberger S, Krocher O, Tissler A, Althoff R. The state of the art in selective catalytic reduction of NO x by ammonia using metal-exchanged zeolite catalysts. Catal Rev. 2008;50(4):492–531.
Brandhorst M, Zajac J, Jones DJ, Roziere J, Womes M, Jimenez- López A. Cobalt-, copper- and iron-containing monolithic aluminosilicate-supported preparations for selective catalytic reduction of NO with NH3 at low temperatures. Appl Catal B: Environ. 2005;55(4):267–76.
Corma A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev. 1997;97(6):2373–420.
Wang YL, Huang ZG, Liu Z, Qingya L. A novel activated carbon honeycomb catalyst for simultaneous SO2 and NO removal at low temperatures. Carbon. 2004;42:445–8.
Pasel J, Kabner P, Montanari B, Gazzano M, Vaccari A, Makowski W, Lojewski T, Dziembaj R, Papp H. Transition metal oxides supported on active carbons as low temperature catalysts for the selective catalytic reduction (SCR) of NO with NH3. Appl Catal B: Environ. 1998;18:199–213.
Zhu Z, Liu Z, Niu H, Liu S, Hu T, Liu T, Xie Y. Mechanism of SO2 promotion for NO reduction with NH3 over activated carbon-supported vanadium oxide catalyst. J Catal. 2001;197:6–16.
Ma J, Liu Z, Liu Q, Guo S, Huang Z, Xiao Y. SO2 and NO removal from flue gas over V2O5/AC at lower temperatures—role of V2O5 on SO2 removal. Fuel Process Technol. 2008;89(3):242–8.
Yanli W, Zhanggen H, Zhengyu L, Qingya L. A novel activated carbon honeycomb catalyst for simultaneous SO2 and NO removal at low temperatures. Carbon. 2004;42:423–60.
Yanli W, Zhenyu L, Liang Z, Zhanggen H, Qingya L, Jianrong M. Performance of an activated carbon honeycomb supported V2O5 catalyst in simultaneous SO2 and NO removal. Chem Eng Sci. 2004;59:5238–90.
Tseng HH, Wey MY, Liang YS, Chen KH. Catalytic removal of SO2, NO and HCl from incineration flue gas over activated carbon-supported metal oxides. Carbon. 2003;41:1079–85.
Wang Y, Liu Z, Zhan L, Huang Z, Liu Q, Ma J. Performance of an activated carbon honeycomb supported V2O5 catalyst in simultaneous SO2 and NO removal. Chem Eng Sci. 2004;59:5283–90.
Namasivayam C, Muniasamy N, Gayathri K, Rani M, Ranganathan K. Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour Technol. 1996;57:37–43.
Sivaraj R, Namasivayam C, Kadirvelu K. Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions. Waste Manage. 2001;21:105–10.
Giraldo L, Moreno-Piraján JC. Activated carbon prepared from orange peels coated with titanium oxide nanoparticles: characterization and applications in the decomposition of NO x . Orient J Chem. 2014;30(2):451–61.
Dumesic JA, Cardona-Martinez N. Applications of adsorption microcalorimetry to the study of heterogeneous catalysis. Adv Catal. 1992;38:149–57.
Wunder RW, Phillips J. Structure of bimetallic particles: nonequimolar graphite-supported Fe–Pd. J Phys Chem. 1996;100:14430–6.
Phillips J, Xia B, Angel-Menéndez J. Calorimetric study of oxygen adsorption on activated carbón. Thermochim Acta. 1998;312(1–2):87–93.
Gatte RR, Phillips J. Microcalorimetric study of the progressive oxidation of the surface of graphite-supported iron microcrystals. Langmuir. 1989;5:758–66.
Gow AS, Phillips J. Microcalorimetric study of reactive surface area on demineralized coal chars. Energy Fuels. 1993;7:674–9.
O’Neil M, Lovrien R, Phillips J. New microcalorimeter for the measurement of differential heats of adsorption of gases on high surface area solids. Rev Sci Instrum. 1985;56:2312–24.
Gravelle PC. Heat-flow microcalorimetry and its application to heterogeneous catalysis. Adv Catal. 1972;22:191–263.
Sumathi S. Removal of SO2 and NO from simulated flue gas using palm shell activated carbon. Ph.D. Thesis, Universiti Sains Malaysia, Malaysia, 2010.
Marsh H, Rodríguez-Reinoso F. Characterization of activated carbon. In: Marsh H, Reinoso F, editors. Activated carbon. Elsevier Science Ltd. United Kingdom. 2005; pp. 157–164.
Moreno-Piraján JC, García-Cuello VS, Giraldo L. Characterization of mordenite-supported Pd, Pt, and Ir determined by CO adsorption microcalorimetry and the dehydrogenation reaction of C3 alkanes. Top Catal. 2011;54:146–52.
Colthup NB, Daly LH, Wiberley SE. Introduction to infrared and Raman spectroscopy. 3rd ed. San Diego: Academic Press; 1990.
Jensen H, Pedersen JH, Jorgensen JE, Pedersen JS, Joensen KD, Iversen SB, et al. Determination of size distributions in nanosized powders by TEM, XRD, and SAXS. J Exp Nanoscience. 2006;1:355–73.
Spirewak BE, Dumesic JA. Applications of adsorption microcalorimetry for the characterization of metal-based catalyst. Thermochim Acta. 1998;312:95–104.
Sousa JPS, Pereira MFR, Figueiredo JL. Carbon xerogel catalyst for NO oxidation. Catalysts. 2012;2:447–65.
Yi H, Deng H, Tang X, Yu Q, Zhou X, Liu H. Adsorption equilibrium and kinetics for SO2, NO, CO2 on zeolites FAU and LTA. J Hazard Mater. 2012;203–204:111–7.
Acknowledgements
The authors would like to thank the Universidad de los Andes (Bogotá, Colombia) and the Universidad Nacional de Colombia (Sede Bogotá), according to the framework agreement between the two institutions under which this research was developed. Special thanks go to the Facultad de Ciencias and the Vice-Rectoría de Investigaciones at the Universidad de los Andes (Bogotá, Colombia) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giraldo, L., Moreno-Piraján, J.C. Adsorption microcalorimetry. J Therm Anal Calorim 121, 245–255 (2015). https://doi.org/10.1007/s10973-015-4684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4684-z