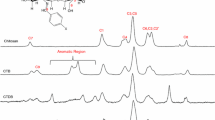
Abstract
Thermal degradation of hydroxypropyl trimethyl ammonium chloride chitosan–Cd complexes (HTCC–Cd) was investigated by thermogravimetric analysis. The results indicate that the degradation of HTCC–Cd in nitrogen atmosphere was two-step reaction. For the first step of degradation, the initial temperature of mass loss (T 0), the final temperature of mass loss (T f), and the temperature of maximum mass loss (T p) increase linearly with the rising of heating rate (B). T o = 1.241B + 220.3, T p = 1.111B + 245.8, and T f = 1.335B + 358.2. Using different methods, the kinetic parameters of the two steps were investigated. The results show that the activation energies of the first step of degradation obtained using Friedman and Flynn–Wall–Ozawa methods are 1.684 × 105 and 1.646 × 105 J mol−1, and the corresponding activation energies for the second step are 1.165 × 105 J mol−1 and 1.373 × 105 kJ mol−1. The results obtained from Phadnis–Deshpande methods indicate that the two degradation processes are both nucleation and growth process, and follow A4 mechanism with intergral form g(X) = [−ln(1 − X)]4.







Similar content being viewed by others
References
Li DH, Liu LM, Tian KL, Liu JC, Fan XQ. Synthesis, biodegradability and cytotoxicity of water-soluble isobutylchitosan. Carbohydr Polym. 2007;67:40–5.
Velyana G, Dilyana Z, Lyubomir V. Non-isothermal kinetics of thermal degradation of chitin. J Therm Anal Calorim. 2013;111:763–71.
Taboada E, Cabrera G, Cardenas G. Retention capacity of chitosan for copper and mercury ions. J Chil Chem Soc. 2003;48:7–12.
Cardenas G, Cabrera G, Taboada E, Miranda SP. Chitin characterization by SEM, FTIR, XRD, and C-13 cross polarization/mass angle spinning NMR. J Appl Polym Sci. 2004;93:1876–85.
Guibal E. Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci. 2005;30:71–109.
Trimukhe KD, Varma AJ. A morphological study of heavy metal complexes of chitosan and crosslinked chitosans by SEM and WAXRD. Carbohydr Polym. 2008;71:698–702.
Taboada E, Cabrera G, Jimenez R, Cardenas G. A kinetic study of the thermal degradation of chitosan-metal complexes. J Appl Polym Sci. 2009;114:2043–52.
Wen YM, Yang L, Li SD. Synthesis of chitosan-schiff base Cu(II) complexes and its application as catalyst in decomposition of H2O2. J Polym Mater. 2009;26:341–50.
Bhatnagar A, Sillanpää M. Applications of chitin and chitosan-derivatives for the detoxification of water and wastewater-a short review. Adv Colloid Interface Sci. 2009;152:26–38.
Zhao Y, Tian JS, Qi XH, Han ZN, Zhuang YY, He LN. Quaternary ammonium salt-functionalized chitosan: a easily recyclable catalyst for efficient synthesis of cyclic carbonates from epoxides and carbon dioxide. J Mol Catal A Chem. 2007;271:284–9.
Zhao Y, Qi XH, He LN, Han ZN, Zhuang YY. Synthesis of propylene carbonate from CO2 and propylene oxide catalyzed by quaternized chitosan. Petrochem Technol. 2007;36:1148–51.
Zhao Y, He LN, Zhuang YY, Wang JQ. Dimethyl carbonate synthesis via transesterification catalyzed by quaternary ammonium salt functionalized chitosan. Chin Chem Lett. 2008;19:286–90.
Lang G, Wendel H, Konrad E, Wella A. U.S. Patent 4921949. 1990.
Huang RH, Chen GH, Sun MK, Hu YM, Gao CJ. Studies on nanofiltration membrane formed by diisocyanate cross-linking of quaternized chitosan on poly(acrylonitrile) (PAN) support. J Membr Sci. 2006;286:237–44.
Li HB, Du YM, Wu XJ, Zhan HY. Effect of molecular mass and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids Surf A Physicochem Eng Asp. 2004;242:1–8.
Li XG, Huang MR. Thermal decomposition kinetics of thermotropic poly(oxybenzoate-co-oxynaphthoate) Vectra copolyester. Polym Degrad Stab. 1999;64:81–90.
Popescu C. Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions a variant on the OzawaeFlynneWall method. Thermochim Acta. 1996;285:309–23.
Dowdy DR. Meaningful activation energies for complex systems-I. The application of the Ozawa-Flynn-Wall method to multiple reactions. J Therm Anal. 1987;32:137–47.
Liu BY, Zhao XJ, Wang XH, Wang FS. Thermal degradation kinetics of poly(propylene carbonate) obtained from the copolymerization of carbon dioxide and propylene oxide. J Appl Polym Sci. 2003;90:947–53.
Sun JT, Huang YD, Gong GF, Cao HL. Thermal degradation kinetics of poly(methylphenylsiloxane) containing methacryloyl groups. Polym Degrad Stab. 2006;91:339–46.
Ou CY, Li SD, Li CP, Zhang CH, Yang L, Chen CP. Effect of cupric ion on thermal degradation of chitosan. J Appl Polym Sci. 2008;109:957–62.
Britto D, Assis OBG. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int J Biol Macromol. 2007;41:198–203.
Vachoud L, Chen TH, Payne GF, Vazquez-Duhalt R. Peroxidase catalyzed grafting of gallate esters onto the polysaccharide chitosan. Enzyme Microb Technol. 2001;29(6–7):380–5.
Julkapli NM, Akil HM, Ahmad Z. Thermal properties of 4,4-oxydiphathalic anhydride chitosan filled chitosan bio-composites. J Therm Anal Calorim. 2012;107:365–76.
Fernanda SP, da Deuber Lincon SA, Aldo EJ, Eduardo RPG. Thermal studies of chitin-chitosan derivatives. J Therm Anal Calorim. 2013;114:321–7.
Jia ZS, Shen DF, Xu WL. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001;333(1):1–6.
Mi FL, Shyu SS, Chen CT, Schoung JY. Porous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: preparation of antigen-adsorbed microsphere and in vitro release. Biomaterials. 1999;20(17):1603–12.
Li SD, Zhang CH, Dong JJ, Ou CY, Quan WY, Yang L, She XD. Effect of cupric ion on thermal degradation of quaternized chitosan. Carbohydr Polym. 2010;81(2):182–7.
Ou CY, Zhang CH, Li SD, Yang L, Dong JJ, Mo XL, Zeng MT. Thermal degradation kinetics of chitosan–cobalt complex as studied by thermogravimetric analysis. Carbohydr Polym. 2010;82:1284–9.
López FA, Mercê ALR, Alguacil FJ, López-Delgado A. A kinetic study on the thermal behaviour of chitosan. J Therm Anal Calorim. 2008;91(2):633–9.
Acknowledgements
The authors would like to acknowledge the financial support by the Chinese National Natural Science Foundation (No. 31271938), the Fundamental Research Funds for Rubber Research Institute, CATAS (No. 1630022013019) and the Fundamental Research Funds from the Environment and Plant Protection Institute, CATAS (No. 2012hzs1J016).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, SD., Li, PW., Yang, ZM. et al. Thermal degradation of hydroxypropyl trimethyl ammonium chloride chitosan–Cd complexes. J Therm Anal Calorim 118, 15–21 (2014). https://doi.org/10.1007/s10973-014-3960-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3960-7