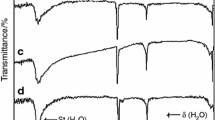
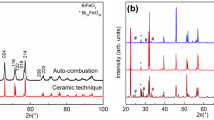
Abstract
The Bi2Fe2(C2O4)5·5H2O was synthesized by solid-state reaction at low heat using Bi(NO3)3·5H2O, FeSO4·7H2O, and Na2C2O4 as raw materials. The nanocrystalline BiFeO3 was obtained by calcining Bi2Fe2(C2O4)5·5H2O at 600 °C in air. The precursor and its calcined products were characterized by thermogravimetry and differential scanning calorimetry, FT-IR, X-ray powder diffraction, and vibrating sample magnetometer. The data showed that highly crystallized BiFeO3 with hexagonal structure [space group R3c(161)] was obtained when the precursor was calcined at 600 °C in air for 1.5 h. The thermal process of the precursor in air experienced five steps which involved, at first, the dehydration of an adsorption water molecule, then dehydration of four crystal water molecules, decomposition of FeC2O4 into Fe2O3, decomposition of Bi2(C2O4)3 into Bi2O3, and at last, reaction of Bi2O3 and Fe2O3 into hexagonal BiFeO3. Based on Starink equation, the values of the activation energies associated with the thermal process of Bi2Fe2(C2O4)5·5H2O were determined. Besides, the most probable mechanism functions and thermodynamic functions (ΔS ≠, ΔH ≠, and ΔG ≠) of thermal processes of Bi2Fe2(C2O4)5·5H2O were also determined.





Similar content being viewed by others
References
Hill NA. Why are there so few magnetic ferroelectrics? J Phys Chem B. 2000;104:6694–709.
Hur N, Park S, Sharma PA, Ahn JA, Guha S, Cheong SW. Electric polarization reversal and memory in a multiferroic material induced by magnetic fields. Nature. 2004;429:392–5.
Zhu WM, Ye ZG. Effects of chemical modification on the electrical properties of 0.67BiFeO3–0.33PbTiO3 ferroelectric ceramics. Ceram Int. 2004;30:1435–42.
Seshadri R, Hill NA. Visualizing the role of Bi 6s “lone pairs” in the off-center distortion in ferromagnetic BiMnO3. Chem Mater. 2001;13:2892–9.
Navarro MC, Lagarrigue MC, De Paoli JM, Carbonio RE, Gómez MI. A new method of synthesis of BiFeO3 prepared by thermal decomposition of Bi[Fe(CN)6]·4H2O. J Therm Anal Calorim. 2010;102:655–60.
Moreira dos Santos A, Parashar S, Raju AR, Zhao YS, Cheetham AK, Rao CNR. Evidence for the likely occurrence of magneto-ferroelectricity in the simple perovskite, BiMnO3. Solid State Commun. 2002;122:49–52.
Yuan GL, Or SW, Wang YP, Liu ZG, Liu JM. Preparation and multi-properties of insulated single-phase BiFeO3 ceramics. Solid State Commun. 2006;138:76–81.
Yun KY, Noda M, Okuyama M. Structural and multiferroic properties of BiFeO3 thin films at room temperature. J Appl Phys. 2004;96:3399–403.
Van Aken BB, Palstra TTM, Filippetti A, Spaldin NA. The origin of ferroelectricity in magnetoelectric YMnO3. Nat Mater. 2004;3:164–70.
Yang CH, Koo TY, Jeong YH. Orbital order, magnetism, and ferroelectricity of multiferroic BiMnO3. J Magn Magn Mater. 2007;310:1168–70.
Choi KJ, Biegalski M, Li YL, Sharan A, Schubert J, Uecker R, Reiche P, Chen YB, Pan XO, Gopalan V, Chen LQ, Schlom DG, Eom CB. Enhancement of ferroelectricity in strained BaTiO3 thin films. Science. 2004;306:1005–9.
Michel C, Moreau JM, Achenbach GD, Gerson R, James WJ. The atomic structure of BiFeO3. Solid State Commun. 1969;7:701–4.
Smolenskii GA, Isupov VA, Agranovskaya AI, Krainik NN. New ferroelectrics of complex composition. Sov Phys Solid State. 1961;2:2651–4.
Smolenskii GA, Yudin VM, Sher ES, Stolypin YE. Antiferromagnetic properties of some perovskites. Sov Phys JETP. 1963;16:622–4.
Moreau JM, Michel C, Gerson R, James WJ. Ferroelectric BiFeO3 X-ray and neutron diffraction study. J Phys Chem Solids. 1971;32:1315–20.
Bucci JD, Robertson BK, James WJ. The precision determination of the lattice parameters and the coefficients of thermal expansion of BiFeO3. J Appl Crystallogr. 1972;5:187–91.
Kubel F, Schmid H. Structure of a ferroelectric and ferroelastic monodomain crystal of the perovskite BiFeO3. Acta Crystallogr. 1990;46:698–702.
Palkar VR, Pinto R. BiFeO3 thin films: novel effects. J Phys. 2002;58:1003–8.
Wang YP, Zhou L, Zhang MF, Chen XY, Liu JM, Liu ZG. Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl Phys Lett. 2004;84:1731–3.
Park TJ, Papaefthymiou GC, Viescas AJ, Moodenbaugh AR, Wong SS. Size-dependent magnetic properties of single-crystalline multiferroic BiFeO3 nanoparticles. Nano Lett. 2007;7:766–72.
Mazumder R, Sujatha Devi P, Bhattacharya Dipten, Choudhury P, Sen A, Raja M. Ferromagnetism in nanoscale BiFeO3. Appl Phys Lett. 2007;91:062510–2.
Lebeugle D, Colson D, Forget A, Viret M. Very large spontaneous electric polarization in BiFeO3 single crystals at room temperature and its evolution under cycling fields. Appl Phys Lett. 2007;91:022907–9.
Lee YH, Wu JM, Lai CH. Influence of La doping in multiferroic properties of BiFeO3 thin films. Appl Phys Lett. 2006;88:042903–5.
Selbach SM, Tybell T, Einarsrud MA, Grande T. Phase transitions, electrical conductivity and chemical stability of BiFeO3 at high temperatures. J Solid State Chem. 2010;183:1205–8.
Ke H, Wang W, Wang YB, Xu JH, Jia DC, Lu Z, Zhou Y. Factors controlling pure-phase multiferroic BiFeO3 powders synthesized by chemical co-precipitation. J Alloy Compd. 2011;509:2192–7.
Szafraniak I, Polomska M, Hilczer B, Pietraszko A, Kepiński L. Characterization of BiFeO3 nanopowder obtained by mechanochemical synthesis. J Eur Ceram Soc. 2007;27:4399–402.
Das N, Majumdar R, Sen A, Maiti HS. Nanosized bismuth ferrite powder prepared through sonochemical and microemulsion techniques. Mater Lett. 2007;61:2100–4.
Basu S, Pal M, Chakravorty D. Magnetic properties of hydrothermally synthesized BiFeO3 nanoparticles. J Magn Magn Mater. 2008;320:3361–5.
Carvalho TT, Tavares PB. Synthesis and thermodynamic stability of multiferroic BiFeO3. Mater Lett. 2008;62:3984–6.
Ghosh S, Dasgupta S, Sen A, Maiti HS. Low temperature synthesis of bismuth ferrite nanoparticles by a ferrioxalate precursor method. Mater Res Bull. 2005;40:2073–9.
Jia DC, Xu JH, Ke H, Wang W, Zhou Y. Structure and multiferroic properties of BiFeO3 powders. J Eur Ceram Soc. 2009;29:3099–103.
Popa M, Crespo D, Calderon-Moreno JM, Preda S. Synthesis and structural characterization of single-phase BiFeO3 powders from a polymeric precursor. J Am Ceram Soc. 2007;90:2723–7.
Wei J, Xue DS. Low-temperature synthesis of BiFeO3 nanoparticles by ethylenediaminetetraacetic acid complexing sol–gel process. Mater Res Bull. 2008;43:3368–73.
Xian T, Yang H, Shen X, Jiang JL, Wei ZQ, Feng WJ. Preparation of high-quality BiFeO3 nanopowders via a polyacrylamide gel route. J Alloy Compd. 2009;480:889–92.
He XB, Gao L. Synthesis of pure phase BiFeO3 powders in molten alkali metal nitrates. Ceram Int. 2009;35:975–8.
Wu XH, Wu WW, Cui XM, Liao S. Preparation of nanocrystalline BiFeO3 via a simple and novel method and its kinetics of crystallization. J Therm Anal Calorim. 2012;107:625–32.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrol. 2008;81:253–62.
Liqing L, Donghua C. Application of iso-temperature method of multiple rate to kinetic analysis. J Therm Anal Calorim. 2004;78:283–93.
Singh RK, Yadav A, Narayan A, Chandra M, Verma RK. Thermal, XRD, and magnetization studies on ZnAl2O4 and NiAl2O4 spinels, synthesized by citrate precursor method and annealed at 450 and 650 °C. J Therm Anal Calorim. 2012;107:205–10.
Bahmani A, Sellami M, Bettahar N. Synthesis of bismuth mixed oxide by thermal decomposition of a coprecipitate precursor. J Therm Anal Calorim. 2012;107:955–62.
Goel SP, Mehrotra PN. IR and thermal studies on lithium oxomolybdenum (VI) oxalate. J Therm Anal. 1985;30:145–51.
Wu XH, Zhou KW, Wu WW, Cui XM, Li YN. Magnetic properties of nanocrystalline CuFe2O4 and kinetics of thermal decomposition of precursor. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-2104-6.
Budrugeac P, Muşat V, Segal E. Non-isothermal kinetic study on the decomposition of Zn acetate-based sol–gel precursor. J Therm Anal Calorim. 2007;88:699–702.
Wu XH, Wu WW, Li SS, Cui XM, Liao S. Kinetics and thermodynamics of thermal decomposition of NH4NiPO4·6H2O. J Therm Anal Calorim. 2011;103:805–12.
Chaiyo N, Muanghlua R, Niemcharoen S, Boonchom B, Seeharaj P, Vittayakorn N. Non-isothermal kinetics of the thermal decomposition of sodium oxalate Na2C2O4. J Therm Anal Calorim. 2012;107:1023–9.
Turmanova SC, Genieva SD, Dimitrova AS, Vlaev LT. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Express Polym Lett. 2008;2:133–46.
Ioiţescu A, Vlase G, Vlase T, Doca N. Kinetics of decomposition of different acid calcium phosphates. J Therm Anal Calorim. 2007;88:121–5.
Vlase T, Vlase G, Birta N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88:631–5.
Vlaev LT, Georgieva VG, Genieva SD. Products and kinetics of non-isothermal decomposition of vanadium (IV) oxide compounds. J Therm Anal Calorim. 2007;88:805–12.
Boonchom B, Baitahe R, Kongtaweelert S, Vittayakorn N. Kinetics and thermodynamics of zinc phosphate hydrate synthesized by a simple route in aqueous and acetone media. Ind Eng Chem Res. 2010;49:3571–6.
Head C, Smith ACK. Applied physical chemistry. London: McMillan Press; 1982. p. 473.
Sĕsták JJ. Thermodynamical properties of solids. Prague: Academia; 1984.
Young D. Decomposition of solids. Oxford: Pergamon Press; 1966.
Acknowledgements
This study was financially supported by the National Nature Science Foundation of China (Grant no. 21161002) and the Guangxi Nature Science Foundation of China (Grant no. 2011GXNSFA018036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Su, P., Wu, W. et al. Preparation of nanocrystalline BiFeO3 and kinetics of thermal process of precursor. J Therm Anal Calorim 111, 1057–1065 (2013). https://doi.org/10.1007/s10973-012-2524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2524-y