Abstract
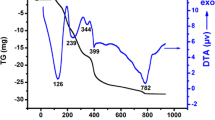
A new perovskite solid solution system of LaCr1−xZnxO3 (0 ≤ x ≤ 0.3) was synthesized through sol–gel method. The effect of Zn doping on the lanthanum chromite prepared was investigated. Thermal decomposition of the dried gel of LaCr0.8Zn0.2O3 was characterized by TG/DTA thermal analysis. The synthesized powders were characterized by means of X-ray diffraction (XRD), infrared spectra (IR), and scanning microscope (SEM). Finally, electrical properties were characterized by the standard four-probe technique. From the preceding analysis, it can be shown that the amorphous powders crystallize in the orthorhombic structure with Pbnm (62) space group (JCPDS card 24-1016), where the crystallite size ranges from 29.46 to 53.21 nm. The oxides LaCr1−xZnxO3 (0 ≤ x ≤ 0.3) have the comportment of semiconductors in the working temperature range 25–350 °C. The electrical conductivity increases with the degree of substitution x, whereas the maximum electrical conductivity obtained is ~13.8 S/cm at 350 °C for LaCr0.3Zn0.3O3 where the electrical conduction occurs by a thermal activated of small polarons hopping. At higher temperature, the electrical behavior is similar to that of pure metal.
Graphical Abstract














Similar content being viewed by others
References
Ishihara T (2009) Perovskite oxide for solid oxide fuel cells. Springer, New York
Chakraborty A, Basu RN, Maiti HS (2000) Mater Lett 45:162–166
Rivas-Vázquez LP, Rendón-Angeles JC, Rodríguez-Galicia JL, Gutiérrez-Chavarria CA, Zhu KJ, Yanagisawa K (2006) J Eur Ceram Soc 26:81–88
Onuma S, Miyoshi S, Yashiro K, Kaimai A, Kawamura K, Nigara Y, Kawada T, Mizusaki J, Sakai N, Yokokawa H (2003) J Solid State Chem 170:68–74
Mori M, Yamamoto T, Itoh H, Watanabe T (1997) J Mater Sci 32:2423–2431
Sfeir J, Van Herle J, McEvoy AJ (1999) J Eur Ceram Soc 19:897–902
Adaika K, Omari M (2015) J Sol–Gel Sci Technol 75:298–304
Larsen PH, Hendriksen PV, Mogensen M (1997) J Therm Anal Calorim 49:1263–1275
Oishi M, Yashiro K, Hong JO, Nigara Y, Kawada T, Mizusaki J (2007) Solid State Ionics 178:307–312
Seyfi B, Baghalha M, Kazemian H (2009) Chem Eng J 148:306–311
Rida K, Benabbas A, Bouremmad F, Peña MA, Martínez-Arias A (2006) Catal Comm 7:963–968
Onuma S, Miyoshi S, Yashiro K, Kaimai A, Kawamura K, Nigara Y, Kawada T, Mizusaki J, Sakai N, Yokokawa H (2003) J Solid State Chem 170:68–74
Shu J, Kaliaguine S (1998) Appl Catal B-Environ 16:303–308
Labhsetwar NK, Watanabe A, Mitsuhashi T (2003) Appl Catal B-Environ 40:21–30
Royer S, Berube F, Kaliaguine S (2005) Appl Catal A-Gen 282:273–284
Nithya VD, Immanuel RJ, Senthilkumar ST, Sanjeeviraja C, Perelshtein I, Zitoun D, Selvan RK (2012) Mat Res Bull 47:1861–1868
Ivanova S, Senyshyn A, Zhecheva E, Tenchev K, Stoyanova R, Fuessb H (2010) J Solid State Chem 183:940–950
Samat AA, Abdullah NA, Ishak MAM, Osman N (2012) World Acad Sc Eng Techol. 6(10):951–955
Hsiang HI, Yen FS, Chang YH (1996) J Mate Sci 31:2417–2424
Diafi M, Omari M (2012) Bol Soc Esp Ceram Vidr 51(6):337–342
Yazdanbakhsh M, Tavakkoli H, Hosseini SM (2011) S Afr J Chem 64:71–78
Baranauskas A, Jasaitis D, Kareiva A (2002) Vibr Spectrosc 28:263–275
Zhenxing Y, Ji Z, Longtu L, Hongguo Z, Zhilun G (2000) J Magn Magn Mat 208:55–56
Lobree LJ, Ch Hwang I, Reimer JA, Bell AT (1999) Catal Lett 63:233–240
Hadjiivanov K, Knozingera H, Tsyntsarskib B, Dimitrov L (1999) Catal Lett 62:35–40
Busca G, Lorenzelli V (1982) Mater. Chem 7:89–126
Mali A, Ataie A (2005) Scr Mater 53:1065–1070
Yu HF, Lin HY, Magn J (2004) Magn Mater 283:190–198
Chakrabarti N, Maiti HS (1997) Mater Lett 30:169–173
Subba Rao GV, Rao CNR, Ferraro JR (1970) Appl Spectrosc 24:436–445
Jitaru I, Berger D, Fruth V, Novac A, Stanica N, Rusu F (2000) Ceram Inter 26:193–196
Zheng W, Pang W, Meng G, Peng D (1999) J Mater Chem 9:2833–2836
Fu YP, Wang HCh, Ouyang J (2011) Int J Hydrogen Energy 36:13073–13082
Jiang SP, Liu L, Ong KP, Wu P, Li J, Pu J (2008) J Power Sources 176:82–89
Mitchell BS (2004) An introduction to materials engineering and science for chemical and meterials engineers. Wiley, Canada
Fu YP, Wang HC (2011) Int J Hydrog Energy 36:747–754
Kang M, Yun J, Cho C, Kim C, Tai W (2013) J Inorg Non-Metall Mat 3:37–42
Tian C, Chan SW (2000) Solid State Ionics 134:89–102
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Solid State Ionics 76:273–283
Xu Q, Huang DP, Chen W, Lee JH, Wang H, Yuan RZ (2004) Scr Mater 50:165–170
Lu S, Yu B, Meng X, Zhao X, Ji Y, Fu C, Zhang Y, Yang L, Fan H, Yang J (2015) J Power Sources 273:244
Makhloufi S, Omari M (2016) J Inorg Organomet Polym 26:32–40
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chadli, I., Omari, M., Abu Dalo, M. et al. Preparation by sol–gel method and characterization of Zn-doped LaCrO3 perovskite. J Sol-Gel Sci Technol 80, 598–605 (2016). https://doi.org/10.1007/s10971-016-4170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4170-5