Abstract
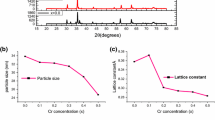
Cr2O3 and MnCr2O4 spinel chromite nanoparticles were synthesized using chemically derived sol–gel technique. Crystal structure was analyzed using X-ray diffraction, and phase transition from a rhombohedral symmetry (R-3c) for Cr2O3 to a spinel cubic symmetry (Fd3 m) for MnCr2O4 has been observed. Data obtained from diffraction were also utilized to evaluate the lattice parameters, crystallite size and unit cell volume. Micrographs obtained using a field emission scanning electron microscope exhibited well-shaped, homogenously distributed 30–70-nm-sized nanoparticles, with well-defined grain. Stoichiometric composition of all the elements present in the samples was confirmed using energy-dispersive X-ray spectroscopy. Dynamic light scattering measurement was performed to corroborate the hydrodynamic diameter and distribution of Cr2O3 and MnCr2O4 nanoparticles. The magnetic behavior of samples was scrutinized as a function of temperature and applied field. It was observed that Cr2O3 exhibited paramagnetic behavior both at room temperature and at 5 K, while a magnetic phase transition from ferro to para was observed in MnCr2O4 with a Curie temperature, T c ~ 50 K.
Graphical Abstract









Similar content being viewed by others
References
Sun B, Wu J, Jia X, Lou F, Chen P (2015) Preparation and light-controlled resistive switching memory behavior of CuCr2O4. J Sol-Gel Sci Technol 75:664–669
Saha D, Giri R, Mistry KK, Sengupta K (2005) Magnesium chromate–TiO2 spinel tape cast thick film as humidity sensor. Sens Actuators B Chem 107:323–331
Geng Q, Zhao X, Gao X, Yang S, Liu G (2012) Low-temperature combustion synthesis of CuCr2O4 spinel powder for spectrally selective paints. J Sol-Gel Sci Technol 61:281–288
Paul B, Bhuyan B, Purkayastha DD, Dhar SS, Behera S (2015) Facile synthesis of spinel CuCr2O4 nanoparticles and studies of their photo catalytic activity in degradation of some selected organic dyes. J Alloys Compd 648:629–635
Ahmad SS, Rhamdhani M, Pownceby M, Bruckard W (2016) Selective sulfidising roasting for the removal of chrome spinel impurities from weathered ilmenite ore. Int J Miner Process 146:29–37
Kim D, Lhm S (2001) Application of spinel-type cobalt chromite as a novel catalyst for combustion of chlorinated organic pollutants. Environ Sci Technol 35:222–226
Li H, Chen W (2010) Stability of MnCr2O4 spinel and Cr2O3 in high temperature carbonaceous environments with varied oxygen partial pressures. Corros Sci 52:2481–2488
Lau K, Kandalam A, Costales A, Pandey R (2004) Equilibrium geometry and electron detachment energies of anionic Cr2O4, Cr2O5, and Cr2O6 clusters. Chem Phys Lett 393:112–117
Jankovsky O, Sedmidubsky D, Sofer Z, Luxa J, Bartunek V (2015) Simple synthesis of Cr2O3 nanoparticles with a tunable particle size. Ceram Int 41:4644–4650
Tong J, Cai X, Wang H, Xia C (2013) Efficient magnetic CoFe2O4 nanocrystal catalyst for aerobic oxidation of cyclohexane prepared by sol–gel auto-combustion method: effects of catalyst preparation parameters. J Sol-Gel Sci Technol 66:452–459
Masrour R, Hamedoun M, Benyoussef A (2010) Magnetic properties of MnCr2O4 nanoparticle. J Magn Magn Mater 322:301–304
Peelamedu R, Grimes C, Agrawal D, Roy R, Yadoji P (2003) Ultralow dielectric constant nickel–zinc ferrites using microwave sintering. J Mater Res 18:2292–2295
Hossain AKMA, Seki M, Kawai T, Tabata H (2004) Colossal magneto resistance in spinel type Zn1−xNixFe2O4. J Appl Phys 96:1273–1275
Zhou Y, Yang Z, Li L, Xie Y, Lin S, Sun Y, Zhang Y (2012) Magnetic field and external pressure effects on the spiral order of polycrystalline MnCr2O4. J Magn Magn Mater 324:3799–3801
Song S, Yuan Z (2003) Electrical properties of MnCr2O4 spinel. J Mater Sci Lett 22:755–757
Mufti N, Blake GR, Palstra TTM (2009) Magneto dielectric coupling in MnCr2O4 spinel. J Magn Magn Mater 321:1767–1769
Yazdanbakhsha M, Khosravi I, Goharshadi G, Youssefi A (2010) Fabrication of nano spinel ZnCr2O4 using sol–gel method and its application on removal of azo dye from aqueous solution. J Hazard Mater 184:684–689
Gao Z, Cui F, Zeng S, Guo L, Shi J (2010) A high surface area super paramagnetic mesoporous spinel ferrite synthesized by a template-free approach and its adsorptive property. Microporous Mesoporous Mater 132:188–195
Sivakumar P, Ramesh R, Ramanand A, Ponnusamy S, Muthamizhchelvan C (2013) Synthesis and characterization of NiFe2O4 nanoparticles and nanorods. J Alloys Compd 563:6–11
Matulkova I, Holec P, Pacakova B, Kubickova S, Mantlikova A, Plocek J, Nemec I, Niznansky D, Vejpravova J (2015) On preparation of nano crystalline chromites by co-precipitation and autocombustion methods. Mater Sci Eng B 195:66–73
Marinkovic Z, Mancic L, Maric R, Milosevic O (2001) Preparation of nanostructure Zn–Cr–O spinel powders by ultrasonic spray pyrolysis. J Eur Ceram Soc 21:2051–2055
Levy S, Diella D, Pavese V, Dapiaggi A, Sani M (2005) P-V equation of state, thermal expansion and P–T stability of synthetic (ZnCr2O4 spinel). Am Miner 90:1157–1167
Hilczer A, Kowalska K, Markiewicz E, Pietraszko A, Andrzejewski B (2016) Dielectric and magnetic response of SrFe12O19–CoFe2O4 composites obtained by solid state reaction. Mater Sci Eng B Solid 207:47–55
Marinkovic ZV, Mancic L, Vulic P, Milosevi O (2005) Microstructure characterization of mechanically activated ZnO–Cr2O3 system. J Eur Ceram Soc 25:2081–2093
Bayhan M, Hashemi T, Brinkman A (1997) Sintering and humidity-sensitive behavior of the ZnCr2O4–K2CrO4 ceramic system. J Mater Sci 32:6619–6623
Ghafoor I, Siddiqi SA, Atiq S, Riaz S, Naseem S (2015) Sol–gel synthesis and investigation of structural, electrical and magnetic properties of Pb doped La0.1Bi0.9FeO3 multiferroics. J Sol-Gel Sci Technol 74:352–356
Cullity BD (1977) Elements of X-ray diffraction, 2nd edn. Notre Dame
Carvalho JWJ, Carvalho FAO, Batista T, Santiago PS, Tabak M (2014) Cetyltrimethylammonium chloride (CTAC) effect on the thermal stability of oxy-HbGp: dynamic light scattering (DLS) and small angle X-ray scattering (SAXS) studies. Colloid Surf B 118:14–24
Sobhani A, Niasari M (2013) Synthesis, characterization, optical and magnetic properties of a nickel sulfide series by three different methods. Superlattices Microstruct 59:1–12
Anandan K, Rajendran V (2014) Studies on structural, morphological, magnetic and optical properties of chromium sesquioxide (Cr2O3) nanoparticles: synthesized via facile solvo thermal process by different solvents. Mat Sci Semicon Process 19:136–144
Vollath D, Szabo D, Willis J (1996) Magnetic properties of nano crystalline Cr2O3 synthesized in a microwave plasma. Mater Lett 29:271–279
Pokhrel S, Simion C, Quemener V, Barsan N, Weimar U (2008) Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens Actuators B Chem 133:78–83
Zhang W, Bru E, Zhang Z, Tegus O, Li W, Si P, Geng D, Buschow K (2005) Structure and magnetic properties of Cr nanoparticles and Cr2O3 nanoparticles. Phys B 358:332–338
Jhuang YC, Kuo KM, Chern G (2011) Structural and magnetic characterizations of Mn2CrO4 and MnCr2O4 films on MgO(001) and SrTiO3(001) substrates by molecular beam epitaxy. J Appl Phys 109:07D714
Hastings JM, Corliss LM (1962) Magnetic structure of manganese chromite. Phys Rev 126:556–565
Acknowledgments
Authors are thankful to Higher Education Commission of Pakistan (HEC) for financially supporting this work through research project number NRPU-2471. The authors also extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for funding this Research Group No. RG-1435-004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzal, A., Atiq, S., Saleem, M. et al. Structural and magnetic phase transition of sol–gel-synthesized Cr2O3 and MnCr2O4 nanoparticles. J Sol-Gel Sci Technol 80, 96–102 (2016). https://doi.org/10.1007/s10971-016-4066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4066-4