Abstract
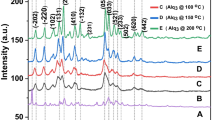
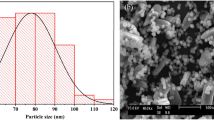
Water solvent-based sol–gel process was employed in the synthesis of nanoparticles of gamma alumina (NPGA) which were used for the removal of fluoride from water of neutral pH. Different techniques like thermogravimetric analysis, Fourier transform infrared spectroscopy, X-ray diffraction, and field emission scanning electron microscopy were employed for the characterization of the synthesized particles. Batch adsorption studies were performed for the optimization of contact time and adsorbent doses to obtain maximum fluoride removal and understanding of the adsorption kinetics and mechanism. The maximum adsorption capacity of NPGA for fluoride removal was estimated to be nearly 14 mg/g at room temperature (30 °C) which is better than fluoride removal reported by earlier using of commercial-grade NPGA. Adsorption kinetics measurement indicated that Langmuir equilibrium model is found to be more suitable for describing the fluoride adsorption mechanism.
Graphical Abstract











Similar content being viewed by others
References
Das DP, Parida K (2003) Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J Colloid Interface Sci 261:213–220
Raichur AM, Basu MJ (2001) Adsorption of fluoride onto mixed rare earth oxides. Sep Purif Technol 24:121–127
Shen F, Chen X, Gao P, Chen G (2003) Electrochemical removal of fluoride ions from industrial wastewater. Chem Eng Sci 58:987–993
World Health Organization (WHO) (2004) Guidelines for drinking-water quality, 3rd edn. WHO, Geneva
Latham MC (1997) Human nutrition in the developing world. Food and Agriculture Organisation, United Rome
Susheela AK (2002) Fluorisis in developing countries: remedial measures and approaches. Proc Indian Natl Sci Acad B68:389–400
Saha S (1993) Treatment of aqueous effluent for fluoride removal. Water Res 27:1347–1350
Amor Z, Mameri BN, Taky M, Nicolas S, Elmidaoui A (2001) Fluoride removal from brackish water by electrodialysis. Desalination 133:215–223
Ghorai S, Pant KK (2004) Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem Eng 981:65–173
Ndiaye PI, Moulin P, Dominguez L, Millet JC, Charbit F (2005) Removal of fluoride from electronic industrial effluent by RO membrane separation. Desalination 173:25–32
Singh IB, Prasad M (2004) Study on fluoride removal characteristic of mineral (fluorapatite). Ind J Chem Technol 11:185–189
Singh IB, Prasad M, Amritphale S (2004) Development of defluridation technology for its easy adaptation in rural areas. J Rural Technol 1:163–166
Daifullah AAM, Yakout SM, Elreefy SA (2007) Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J Hazard Mater 147:633–643
Leyva-Ramos R, Medellin-Castillo NA, Jacobo-Azuara A, Landin Mendoza-Barron J, Rodriguez LE, Martinez-Rosales JM, Aragon-Piña A (2008) Fluoride removal from water solution by adsorption on activated alumina prepared from pseudo-boehmite. J Environ Eng Manage 18:301–309
Fawel J, Bailey K, Chilton J, Dahi E, Fewtrell L, Magara Y (2006) Fluoride in drinking-water. IWA Publishing, London
Onyango MS, Matsuda H (2006) Fluoride removal from water using adsorption technique. In: Tressaud A (ed) Fluorine and the environment. Elsevier, B V, Amsterdam, pp 1–48
Jovancic P, Radetic M, Barcelo D, Petrovic M (eds) (2008) Emerging contaminants from industrial and municipal waste: removal technologies (The handbook of environmental chemistry/water pollution), vol 5. Springer, Berlin, Heidelberg, pp 239–264
Hristovski K, Baumgardner A, Westerhoff P (2007) Selecting metal oxide nano materials for arsenic removal in fixed bed columns: from nanopowders to aggregated nanoparticle media. J Hazard Mater 147:265–274
Kumar E, Bhatnagar A, Kumar U, Sillanp M (2011) Deflouridation from aqueous solution by nano alumina: characterization and sorption studies. J Hazard Mater 186:1042–1049
Puttamraju P, SenGupta AK (2006) Evidence of tunable on–off sorption behaviors of metal oxide nanoparticles: role of ion exchanger support. Ind Eng Chem Res 45:7737–7742
Zhao X, Shi Y, Cai Y, Mou S (2008) Cetyltrimethylammonium bromide-coated magnetic nanoparticles for the preconcentration of phenolic compounds from environmental water samples. Environ Sci Technol 42:1201–1206
Zhao X, Wang J, Wu F, Wang T, Cai Y, Shi Y, Jiang G (2010) Removal of fluoride from aqueous media by Fe3O4 & Al(OH)3 magnetic nanoparticles. J Hazard Mater 173:102–109
Wu M, Lin G, Chen D, Wang G, He D, Feng S, Xu R (2002) Sol hydrothermal synthesis and hydrothermally structural evolution of nano crystalline titanium dioxide. Chem Mater 14:1974–1980
Yanagisawa K, Rendon-Angeles JC, Kanai H, Yamashita Y (2000) Stability and single crystal growth of lead scandium niobate and its solid-solution with lead titanate under hydrothermal conditions. J Mater Sci 35:3011–3015
Yang Q, Li Y, Yin Q, Wang P, Cheng Y-B (2002) Hydrothermal synthesis of bismuth oxide needles. Mater Lett 55:46–49
Rozman M, Drofenik M (1995) Hydrothermal synthesis of manganese zinc ferrite. J Am Ceram Soc 78:2449
Wang CC, Ying JY (1999) Sol–gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem Mater 11:3113–3120
Yoldas BE (1975) Alumina sol preparation from alkoxides. Am Ceram Soc Bull 51:289–290
Singh IB, Ruhi G, Modi OP (2015) Development of sol–gel alumina coating on 9Cr–1Mo steel and their oxidation behavior at high temperature. J Sol-Gel Sci Technol 74:685–691
Ruhi G, Modi OP, Singh IB (2009) Effect of sintering atmosphere on the corrosion and wear resistance of sol–gel alumina coated mild steel surface. Corrosion 65:758–764
Greenberg AE (ed) (1985) Standard methods for the examination of water and waste water. American Public Health Association (APHA), Washington DC
Chena N, Zhang Z, Fenga C, Lia M, Sugiurab N (2011) Investigations on the batch and fixed-bed column performance of fluoride adsorption by Kanuma mud. Desalination 268:76–82
Guzman-Castillo ML, Hernandez-Beltran F, Fripiat JJ, Rodriguez- Hernandez A, Garcia de Leon R, Navarrete-Bolanos J, Tobon-Cervantes A, Bokhimi X (2005) Physicochemical properties of aluminas obtained from different aluminum salts. Catal Today 107–108:874–878
Rinaldi R, Schuchardt U (2005) On the paradox of transition metal-free alumina catalyzed oxidation with aqueous hydrogen peroxide. J Catal 236:335–345
Lippens BC, Boer JH (1964) Study of phase transformations during calcinations of Al hydroxides by selected electron diffraction. Acta Crystallogr 17:1312
Liu Q, Guo H, Shan Y (2010) Adsorption of fluoride on synthetic siderite from aqueous solution. J Fluor Chem 131:635–641
Misra C (1986) Industrial alumina chemicals, ACS Monograph184. American Chemical Society, Washington DC
Sohlberg K, Pennycook SJ, Pantelides ST (1999) Hydrogen and the structure of the transition aluminas. J Am Ceram Soc 121:7493
Frederickson LD (1954) Characterization of hydrated aluminas by infrared spectroscopy: application to study of bauxite ores. Anal Chem 26:1883–1885
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Biochem 34:451–465
Hu CY, Lo SL, Kuan WH (2005) Effects of the molar ratio of hydroxide and fluoride to Al(III) on fluoride removal by coagulation and electro-coagulation. J Colloid Interface Sci 283:472–476
Zhang W, Sun M, Prins R (2002) Multinuclear MAS NMR identification of fluorine species on the surface of fluorinated γ-alumina. J Phys Chem B 106:11805–11809
Acknowledgments
Authors would like to thank Director, CSIR-AMPRI, for providing experimental facilities and also his approval to one of the author (AG) to carry out her internship without any financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, I.B., Gupta, A., Dubey, S. et al. Sol–gel synthesis of nanoparticles of gamma alumina and their application in defluoridation of water. J Sol-Gel Sci Technol 77, 416–422 (2016). https://doi.org/10.1007/s10971-015-3869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3869-z