Abstract
A mesoporous SiO2 template containing TiO2 nanocrystals is synthesized via a sol–gel route, and Au nanoparticles (NPs) are deposited in the tubular mesochannels of the template by a photodeposition method (Au/SiO2–TiO2). Both of the photocatalytic activities under ultraviolet (UV) and visible (Vis) light irradiation are improved by the deposition of Au NPs. A commercially available photocatalyst, Degussa P25 is employed as a reference, and a comparative study shows that Au/SiO2–TiO2 possesses higher photocatalytic activity than that of P25. An action spectrum of methylene blue (MB) photobleaching using Au/SiO2–TiO2 reveals that the photocatalysis of Au/SiO2–TiO2 is carried out by three triggers: UV absorption by TiO2, and Vis light absorptions by Au NPs and MB.
Graphical abstract

Similar content being viewed by others
1 Introduction
TiO2 is generally known as a material that is nontoxic, inexpensive and able to decompose many kinds of organic compounds under sunlight [1]. However, one drawback of photocatalysis by TiO2 is that it can only be excited by ultraviolet (UV) radiation, which occupies only a small portion (3–5 %) of solar radiation [2]. Therefore, an extension of its absorption wavelength range to the visible (Vis) region is an important issue to improve the photocatalytic performance of TiO2. To extend the absorption wavelength, doping with nitrogen [3, 4], carbon [5, 6] or metal ions [7, 8] to TiO2 has been reported. However, the doping produces recombination centers in the TiO2 lattice [9]. Thus, although the photocatalytic response under Vis light irradiation appears, the efficiency under UV radiation unfortunately decreases because the generated electrons and holes easily sustain recombination at the doping center. Therefore, Vis light responsible TiO2 made by element doping would not be appropriate to achieve the photocatalysts with very high activity.
Recently, a modification of TiO2 with metal nanoparticles (NPs) attracts growing attention [10–12]. Two positive effects on the photocatalytic activity have been reported by deposition of metal NPs on TiO2 surface. First, Schottky barrier forms at the interface between TiO2 and noble metal NPs [10–12]. The barrier promotes the interfacial electron transfer from TiO2 to metal NPs and increases the lifetimes of electrons and holes, resulting in the high photocatalytic activity. Second, TiO2–metal NP composite exhibits photocatalytic performance under Vis light irradiation. This is because metal NPs absorb Vis and near-infrared lights due to their surface plasmon resonance (SPR) [13–15]. The excited electrons in the metal NPs transfer to a conduction band of TiO2, resulting in the interfacial separation of electrons and holes that causes photocatalysis [16, 17]. Additionally, since a light absorption coefficient of SPR of metal NPs is far large compared to that of the dopants such as nitrogen, carbon and metal ions [15], TiO2 modified with metal NPs is expected to exhibit high photocatalytic efficiency under both UV and Vis light irradiation.
The photocatalytic performance strongly depends on the surface area of the materials; therefore, the use of porous materials such as mesoporous SiO2 has been studied [18, 19]. Mesoporous SiO2 can improve the degree of dispersion and accessibility of molecules to the photoreactive metal oxides and/or metal NPs [19, 20]. Thus, for example, high photocatalytic activity of TiO2 modified with metal NPs can be further improved by the deposition of metal NPs/TiO2 onto a mesoporous SiO2. Furthermore, the mesoporous SiO2 acts as template to control the size and shape of metal nanoparticles. Metal NPs do not grow over the diameter of mesopores, and metal nanorods (NRs) can be easily produced since metal NPs grow along the mesopores. Many researchers have used mesoporous materials to control the size of metal NPs [21, 22]. However, variation in photocatalytic activity based on the shape of Au nanoparticles has seldom been reported.
We have reported on the synthesis of noble metal NPs deposited onto mesoporous oxide templates [23–25]. Alignment- and length-controlled metal NRs could be obtained by taking advantages of mesoporous SiO2 and SiO2–TiO2 templates [24, 25]. Also, this mesoporous oxide–metal NP composites exhibited the photocatalysis under both UV and Vis light irradiation [25, 26]. In this study, we prepared a mesoporous SiO2 template containing TiO2 nanocrystals, and then deposited Au NPs and Au NRs into the tubular mesochannels of the template (Au/SiO2–TiO2). The photocatalytic properties of Au NPs and NRs/SiO2–TiO2 under UV and Vis light irradiation were investigated by measuring action spectra for the photobleaching of methylene blue to reveal the effects of deposition of Au NPs and NRs.
2 Experimental
2.1 Synthesis of mesoporous SiO2–TiO2 template
Pluronic P123 (1.74 g, triblock copolymer of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide), M w = 5800, EO20PO70EO20, Aldrich, USA) and NaCl (2.92 g, Wako, Japan) were dissolved in 1 M HCl aqueous solution (100 mL, Wako, Japan). The mixture was stirred at room temperature until it became clear. Tetraethoxysilane (4.17 g, Shin-Etsu, Japan) was added and stirred at 35 °C for 24 h. Titanium-n-tetrabutoxide (1.70 g, Wako, Japan) was added to the solution and then stirred for further 6 h. The molar ratio of Si:Ti was 4:1. The solution was maintained at 100 °C for 24 h to allow precipitation. The resulting precipitate was collected by suction filtration, and then washed with ion-exchanged water (IEW) and ethanol (Wako, Japan). The powder was finally calcined at 550 °C for 5 h to remove the surfactant, P123.
2.2 Deposition of Au NPs and NRs on template
The prepared template (0.574 g) was dispersed in a mixture of 1 mM HAuCl4 aqueous solution (2.4 mL, Kishida, Japan), IEW (11.9 mL) and methanol (14.3 mL, Wako, Japan), and then stirred at 25 °C for 1.5 min under UV radiation (USHIO SP-7, 230–440 nm, 93 mW cm−1 at 365 nm) to deposit Au NPs. After the suction filtration and washing with IEW, the powder was dried in an ambient environment. The amount of Au deposited on the template was fixed to be 0.6 mol % (0.6Au/SiO2–TiO2). 0.3, 1.2 and 1.8 mol %Au-deposited powders were also prepared using the same method. In the case of Au NRs (0.6 mol %), light intensity and irradiation time were changed to 30 mW cm−1 and 15 min.
2.3 Characterization of Au/SiO2–TiO2
X-ray diffraction (XRD) patterns were recorded on a Rigaku Ultima IV diffractometer (30 kV, 20 mA, Japan) with CuKα radiation (λ = 1.5406 Å). Transmission electron microscope (TEM) images were taken by a JEOL JEM-2100F microscope at an acceleration voltage of 200 kV (Japan). UV–Vis-NIR diffuse reflectance (DR) spectra were measured with a JASCO V-670 UV–Vis-NIR spectrophotometer (Japan). Surface areas were determined by N2 adsorption isotherms with the Brunauer–Emmett–Teller (BET) method using a Micromeritics TriStar II 3020 adsorption analyzer (USA). The samples were degassed at 150 °C for 2 h in a vacuum prior to the measurement of surface area.
2.4 Evaluation of photocatalytic activity
The photocatalytic activity of Au/SiO2–TiO2 was evaluated by measuring the rate of methylene blue (MB, Wako, Japan) bleaching in aqueous solution under a light illumination. The prepared powder (10 mg) was stirred in an aqueous solution of 1.88 × 10−5 M MB (20 mL) in the dark for 10 min to allow the complete adsorption of MB on the surface of the powder. The suspended powder was then irradiated with UV (<380 nm, 1.8 mW cm−2 at 365 nm) or Vis light (>480 nm, 40 mW cm−2) using an Asahi HAL-320 light source (300 W Xe lamp, Japan) under magnetic stirring. A small portion (1 mL) of the solution was taken after light irradiation for certain periods (5, 10, 15, 20, 25 and 30 min) and centrifuged at 5000 rpm for 5 min to separate from the suspending powder. The concentration of MB in the solution was evaluated by recording the absorbance of MB at 664 nm with a JASCO V-560 UV–Vis spectrophotometer (Japan). For the measurement of action spectra, the MB solution was exposed to monochromatic light (365–820 nm) generated from a 300 W Xe lamp equipped with an Asahi CMS-100 monochromator (full width at half maximum of wavelength = 40 nm, Japan). An apparent quantum yield was calculated as the ratio of the rate of electron consumption by the photobleaching reaction of MB to the flux of incident photons. In practice, the intensity of the incident light was adjusted using neutral density (ND) filter to the photon flux of 7.0 × 10−8 einsteins s−1 at each wavelength.
3 Results and discussion
3.1 Characterization of SiO2–TiO2 and Au/SiO2–TiO2
The XRD pattern and TEM images of an SiO2–TiO2 template are shown in Fig. 1. Peaks of anatase TiO2 were clearly observed as well as a halo of amorphous SiO2 at ca. 23° in the XRD pattern (Fig. 1a). The template possessed an ordered tubular mesoporous structure with a pore diameter of ca. 8 nm that was proved by the TEM image (Fig. 1b). A high-resolution (HR)-TEM image of SiO2–TiO2 template was inserted in Fig. 1b, and the image showed that nanocrystals with a diameter of 2–5 nm were well dispersed in the template. This d spacing of 3.51 Å is consistent with that of the {011} planes of anatase TiO2 [27]. These results show that the mesoporous SiO2–TiO2 template with ordered tubular pores consists of amorphous SiO2 and well-dispersed anatase TiO2 nanocrystals.
The TEM images and DR spectrum of an Au NPs and NRs/SiO2–TiO2 composite are shown in Fig. 2. In Au NPs-deposited sample, spherical NPs with a diameter of 7–15 nm were observed on the inside and outside of the template (Fig. 2a). The inset of Fig. 2a is a HR-TEM image showing that the observed NP is Au NP, because of the d spacing of 2.35 Å [28]. On the other hand, rod-like NPs with a diameter of 7 nm and lengths of 40–100 nm were observed in the mesopores of Au NRs-deposited sample (Fig. 2b). The specific surface areas of SiO2–TiO2, 0.6Au NPs and NRs/SiO2–TiO2 were 518.2, 303.1 and 353.2 m2 g−1, respectively. The decrease in specific surface area is due to the Au blockage in the tubular mesopores. However, the relatively narrow decrease range indicates that there are interpores in this template like SBA-15 [29]. The specific surface area of Au NPs/SiO2–TiO2 was smaller than that of Au NRs/SiO2–TiO2. This is presumably because the number of Au NPs was larger than Au NRs when the same amount of Au atoms was deposited, resulting in effective blocking of the mesopores. The specific surface area of Au/SiO2–TiO2 decreased with increasing the amount of deposited Au because the Au blockage in the tubular mesopores would occur more often. Au NPs/SiO2–TiO2 showed extinctions at shorter wavelength than 380 nm and at ca. 542 nm. On the other hand, Au NRs/SiO2–TiO2 showed extinction peaks at 525 and 712 nm in the DR spectrum (Fig. 2c). In comparison with the spectra of mesoporous SiO2 and SiO2–TiO2 template made by the similar sol–gel method, the extinction below 380 nm was attributed to light absorption by the band gap of anatase TiO2, and the extinction peaks at 542, 525 and 712 nm were defined as the extinction by SPR of the deposited Au NPs and NRs. The wavelength of SPR was well consistent with the size and shape of the deposited Au NPs and NRs [13–15].
3.2 Evaluation of photocatalytic property
The photobleaching dynamics of MB solution with samples before and after Au deposition under UV and Vis light are shown in Fig. 3 and Fig. S1. For comparative purposes, commercially available Degussa P25 (AEROSIL, Japan) was employed, and the results of photobleaching of MB by P25 are also shown in Fig. 3. Adsorption of MB on the samples was saturated by stirring for 10 min before light irradiation. The y axes in Fig. 3 and Fig. S1 denote the absorbance of MB solution after light irradiation for certain time (I x) divided by the initial absorbance of MB solution after adsorption process (I 0). The absorbance of MB decreased by 60, 40, 20, 15 and 8 % by the adsorption process for 10 min when the SiO2–TiO2 and 0.3, 0.6, 1.2 and 1.8Au/SiO2–TiO2 powders were used, respectively. Twenty-five percent decrement of MB absorbance was confirmed with Au NRs/SiO2–TiO2. Meanwhile, the absorbance of MB did not decrease when P25 was used (see the inset of Fig. 3a and Fig. S1a). The differences in the amount of adsorption of MB were in good agreement with the specific surface areas of each powder (see Table S1 and Table 1). The absorbance of MB solution decreased by further 34 % after UV radiation for 30 min when the SiO2–TiO2 powder was used. This value of decrease was almost the same as that of P25. On the other hand, decreases of the absorbance by 75 and 80 % occurred after UV radiation for 30 min when 0.6Au/SiO2–TiO2 and Au NRs/SiO2–TiO2 were used. Decreases of the absorbance by 44, 20 and 14 % occurred after UV radiation when 0.3, 1.2 and 1.8 powders were used, respectively. First-order reaction rate constants of these reactions under UV, k UV are also shown in Table 1 and Table S1. 0.6Au/SiO2–TiO2 showed the highest k UV among the powders with the difference amount of Au. Au NRs/SiO2–TiO2 showed almost the same k UV as 0.6Au/SiO2–TiO2. The rate constants of Au NRs/SiO2–TiO2 and 0.6Au/SiO2–TiO2 were approximately four times larger than those of SiO2–TiO2 and P25. It is worth mentioning here that the surface areas of 0.6Au/SiO2–TiO2 and Au NRs/SiO2–TiO2 were smaller than that of SiO2–TiO2 (303.1, 353.2 and 518.2 m2g−1, respectively). This indicates that the photocatalytic performance of the template per surface area was doubtlessly improved by the deposition of Au. This improvement of the photocatalytic performance by the deposition of Au NPs is presumably due to the fact that the charge separation lifetime is elongated by the formation of a Schottky barrier at the Au–TiO2 interface [10, 11, 30, 31]. However, 1.2 and 1.8Au/SiO2–TiO2 showed lower value than that of the template. This result is presumably due to the large decrease in specific surface area, leading to blockage of access of MB to the inside of the powders. On the other hand, higher photocatalytic activity of Au/SiO2–TiO2 than that of P25 can also be related to the effect of Si–O–Ti bond formed in the mesoporous SiO2–TiO2 template because the existence of Si–O–Ti bonds causes the lowering of both the HOMO and LUMO levels as compared with TiO2 [32].
SiO2–TiO2 template showed a decrease of the absorbance of MB solution by 13 % after Vis light irradiation for 30 min, whereas P25 showed little photocatalytic activity under Vis light (Fig. 3b). These differences in the photocatalytic activity between two samples under Vis light irradiation could be derived from the structural defects that should be contained in the mesoporous template, and the defects can absorb Vis photons [33]. 0.6Au/SiO2–TiO2 composite showed decrease of the absorbance by 25 % after Vis light irradiation for 30 min among samples. From these results, the first-order reaction rate constants under Vis light, k Vis, were calculated, and the value of 1.04 × 10−2 min−1 for 0.6Au/SiO2–TiO2 was approximately two and 16 times higher than those of the SiO2–TiO2 and P25, respectively (see Table 1). Additionally, the photocatalytic activities of 0.3, 1.2 and 1.8Au/SiO2–TiO2 under Vis light were lower than that of 0.6Au/SiO2–TiO2 (Table S1 and Fig. S1b). A deposition of Au onto TiO2 enables SPR-induced photocatalysis under Vis light [16]; however, too much deposition leads to a decrease in surface area and Au blockage in mesopores. On the other hand, we have previously prepared another type of Au/SiO2–TiO2, for which Au was deposited using a heat-reduction method [34]. The Au/SiO2–TiO2 prepared by heat-reduction method exhibited almost no photocatalytic activity because many Au NPs were deposited on SiO2. Conversely, in this work, Au was deposited by a light-reduction method through an excitation of electrons of TiO2, followed by a transfer of the electrons from TiO2 to Au ions. Therefore, Au NPs should be deposited on TiO2, and the interface of Au/TiO2 causes SPR-induced photocatalysis, resulting in the high photocatalytic activity of Au/SiO2–TiO2 in this work. Furthermore, Au NRs/SiO2–TiO2 composited showed even larger decrease of the absorbance of MB solution (35 %) than that of 0.6Au/SiO2–TiO2. This improvement of photocatalytic activity is probably due to the wider absorption wavelength region of SPR by Au NRs. Au NPs showed extinction at 500–600 nm in Vis region. On the other hand, Au NRs showed extinction at 500–800 nm (shown in Fig. 2c). In the next section, the action spectra for the samples were measured to investigate the detailed mechanisms of the photocatalysis.
3.3 Action spectra
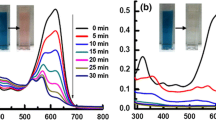
The action spectra of photobleaching of MB with no photocatalyst and mesoporous SiO2–TiO2 before and after Au deposition are shown in Fig. 4. The DR spectra of Au NPs and NRs/SiO2–TiO2 composite and absorption spectrum of MB solution are also shown in the same figure. No photobleaching of MB occurred when MB solution containing no photocatalyst was irradiated with light of any wavelength (closed circles). This indicates that light irradiation itself has no direct influence on photobleaching of MB in the solution. In contrast, photobleaching of MB occurred when the solution with SiO2–TiO2 was exposed to lights of 365 and 600–700 nm (closed triangles). The bleaching occurred presumably because the wavelengths of incident light overlapped with the absorption wavelengths of TiO2 and MB. Since the photobleaching did not occur when the irradiation of light of 600–700 nm was carried out to the pure MB solution, it was found that the adsorption of MB on the surface of SiO2–TiO2 is indispensable to the photobleaching of MB under the Vis light of 600–700 nm, though the structural defects in the mesoporous template would have small contribution to the photobleaching under Vis light irradiation. On the other hand, a peak at 540 nm appeared in the action spectrum of Au NPs/SiO2–TiO2 in addition to the peaks at 365 and 600–700 nm (closed squares). This new peak in the action spectrum and the SPR peak of Au/SiO2–TiO2 in the DR spectrum have comparable wavelengths. Therefore, a trigger of photobleaching caused by light of 540 nm was attributed to the light absorption by SPR of Au NPs. Au NRs/SiO2–TiO2 showed photobleaching of MB when the sample was exposed to lights of 365 and 500–1000 nm (closed reverse triangles). These wavelengths are consistent with the SPR peaks of Au NRs/SiO2–TiO2. Thus, SPR of Au NRs was also found to be able to use for photobleaching of MB. These results concluded that the photobleaching under Vis light irradiation occurs by the light absorption of MB adsorbed on samples and SPR of Au NPs and Au NRs. Au NPs/SiO2–TiO2 can cause Vis light absorption in the range of 500–700 nm and Au NRs/SiO2–TiO2 can cause Vis light absorption in the range of 500–1000 nm, leading to the high thus can show the highest photocatalytic activity under Vis light irradiation.
Action spectra of MB bleaching using MB solution with no samples (filled circle), SiO2–TiO2 (filled triangle), Au NPs/SiO2-TiO2 (filled square) and Au NRs/SiO2-TiO2 (filled inverted triangle). DR spectrum of Au NPs and NRs/SiO2–TiO2 and absorption spectrum of MB solution are also shown as references
In order to further investigate the mechanisms of photocatalysis occurred in this study, changes in absorption spectra of MB solution during the photocatalyses using Au NPs/SiO2–TiO2 under UV or Vis light irradiation were recorded and are shown in Fig. 5. MB in the oxidation state in water shows blue color and is normally bleached by reduction [35]. However, in the case that the bleaching is accompanied with a blue shift in the absorption spectrum, the bleaching of MB is being caused by demethylation instead of reduction [35, 36]. This demethylation of MB occurs because the photocatalytically generated electron-withdrawing groups such as OH radicals and positive holes desorb an electron-releasing methyl group from MB [36]. Since the photobleachings of MB under light of various wavelengths in this study showed relatively little blue shift in the absorption spectra compared to the reported literatures [35, 36], both of the generated electrons and holes by light irradiation should have contributed to the photobleaching of MB. From above consideration, we concluded that there must be following three mechanisms of photobleaching of MB using Au/SiO2–TiO2:
(1) Anatase TiO2 in the SiO2–TiO2 template absorbs UV. The excited electrons in TiO2 are transferred to Au NPs because of the Schottky barrier formed at the interface of Au NPs and TiO2. Photocatalyses occur by the separated electrons and holes on the surfaces of Au NPs and TiO2, respectively (Fig. 6a). (2) Au NPs absorb Vis light of 500–580 nm by SPR. The excited electrons in Au NPs are transferred to the conduction band of TiO2 [16, 37, 38]. Photocatalyses are caused by the separated electrons and holes on the surfaces of TiO2 and Au NPs, respectively (Fig. 6b). Also, SPR wavelength of Au NPs depends on the shape and size. Therefore, Au NPs with wide SPR wavelength region such as Au NRs would show higher activity under Vis light. (3) MB absorbs Vis light of 600–700 nm. The excited electrons in MB that adsorbed on the surface of the sample are transferred to the conduction band of TiO2 [36, 39, 40]. Reduction reaction occurs by the transferred electrons on the surface of TiO2. MB with a lack of electron becomes a cation radical and then is oxidized by O2 − that generated on the surface of TiO2 [39, 40].
4 Conclusions
Mesoporous SiO2–TiO2 was synthesized by the sol–gel method and then Au NPs and NRs were deposited in the tubular mesopores by a photodeposition method. The photocatalytic properties of them under UV and Vis light irradiation were separately measured and compared to that of the commercially available photocatalyst, P25. Au NRs/SiO2–TiO2 showed the highest photocatalytic activity among the samples under both UV and Vis light irradiation. The reasons of the high photocatalytic activity of Au/SiO2–TiO2 were investigated by measuring the action spectra and were considered to be the high specific surface area (353.2 m2g−1), the formation of Schottky barrier between Au NPs and TiO2, the light absorption by SPR of Au NRs and the adsorption of MB on the sample. Moreover, a slight blue shift of the peak of MB in the absorption spectra during the photobleaching revealed that both of the generated electrons and holes on Au/SiO2–TiO2 by light irradiation contributed to the photobleaching of MB. This is also related to the high photocatalytic activity of Au/SiO2–TiO2.
References
Lindner M, Theurich J, Bahnemann DW (1977) Water Sci Technol 35:79–87
Ohtani B (2008) Chem Lett 37:216–229
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269–271
Zaleska A, Gorska P, Sobczak JW, Hupka J (2007) Appl Catal B 76:1–8
Sakthivel S, Kisch H (2003) Angew Chem Int Ed 42:4908–4911
Gorska P, Zaleska A, Kowalska E, Klimczuk T, Sobczak JW, Skwarek E, Janusz W, Hupka J (2008) Appl Catal B 84:440–447
Fox MA, Dulay MT (1993) Chem Rev 93:341–357
Herrmann JM, Disdier J, Pichat P (1984) Chem Phys Lett 108:618–622
Ohtani B, Bowman RM, Kolombo DP, Kominami H, Noguchi H, Uosaki K (1998) Chem Lett 27:579-580
Iliev V, Tomova D, Bilyarska L, Tyuliev G (2007) J Mol Catal A Chem 263:32–38
Bannat I, Wessels K, Oekermann T, Rathousky J, Bahnemann D, Wark M (2009) Chem Mater 21:1645–1653
Centeno MA, Hidalgo MC, Dominguez MI, Navío JA, Odriozola JA (2008) Chem Lett 123:198–206
Huang X, Neretina S, El-Sayed MA (2009) Adv Mater 21:4880–4910
Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM, Mulvaney P (2005) Coordin Chem Rev 249:1870–1901
Wiley BJ, Sun Y, Xia Y (2007) Acc Chem Res 40:1067–1076
Kowalska E, Omar O, Mahaney P, Abe R, Ohtani B (2010) Phys Chem Chem Phys 12:2344–2355
Kochuveedu ST, Kim DP, Kim DH (2012) J Phys Chem C 116:2500–2506
Sahu DR, Hong LY, Wang SC, Huang JL (2009) Microporous Mesoporous Mater 117:640–649
Qiao WT, Zhou GW, Zhang XT, Li TD (2009) Mater Sci Eng C 29:1498–1502
Beck A, Horváth A, Stefler G, Katona R, Geszti O, Tolnai G, Liotta LF, Guczi L (2008) Catal Today 139:180–187
Li Z, Kübel C, Pârvulescu VI, Richards R (2008) ACS Nano 2:1205–1212
Pérez MD, Otal E, Bilmes SA, Soler-Illia GJAA, Crepaldi EL, Grosso D, Sanchez C (2004) Langmuir 20:6879–6886
Kawamura G, Hayashi I, Muto H, Matsuda A (2012) Scr Mater 66:479–482
Kawamura G, Murakami M, Okuno T, Muto H, Matsuda A (2011) RSC Adv 1:584–587
Kawamura G, Okuno T, Muto H, Matsuda A (2012) Nanoscale Res Lett 7:27
Kawamura G, Okuno T, Muto H, Matsuda A (2014) J Nanosci Nanotechnol 14:2225–2230
PDF Card #00-001-0562 PCPDFWIN, Version 2, JCPDS-ICDD, 2009
PDF Card #00-001-0172 PCPDFWIN, Version 2, JCPDS-ICDD, 2009
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Science 279:548–552
Yu K, Tian Y, Tatsuma T (2006) Phys Chem Chem Phys 8:5417–5420
Sakthivel S, Shankar MV, Palanichamy M, Arabindoo B, Bahnemann DW, Murugesan V (2004) Water Res 38:3001–3008
Fujishima M, Takatori H, Tada H (2011) J Colloid Interface Sci 361:628–631
Li D, Ohashi N, Hishita S, Kolodiazhnyi T, Haneda H (2005) J Solid State Chem 178:3293–3302
Okuno T, Kawamura G, Muto H, Matsuda A (2014) J Mater Sci Technol 30:8–12
Zhang T, Oyama T, Aoshima A, Hidaka H, Zhao J, Serpone N (2001) J Photochem Photobiol A 140:163–172
McEvoy JG, Cui W, Zhang Z (2013) Catal Today 207:191–199
Tian Y, Tatsuma T (2004) Chem Commun. doi:10.1039/B405061D
Tian Y, Tatsuma T (2005) J Am Chem Soc 127:7632–7637
Epling GA, Lin C (2002) Chemosphere 46:561–570
Zhang F, Zhao J, Shen T, Hidaka H, Pelizzetti E, Serpone N (1998) Appl Catal B Environ 15:147–156
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okuno, T., Kawamura, G., Muto, H. et al. Three modes of high-efficient photocatalysis using composites of TiO2-nanocrystallite-containing mesoporous SiO2 and Au nanoparticles. J Sol-Gel Sci Technol 74, 748–755 (2015). https://doi.org/10.1007/s10971-015-3658-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3658-8