Abstract
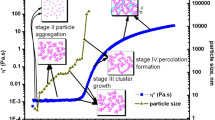
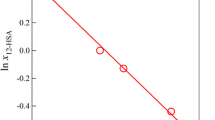
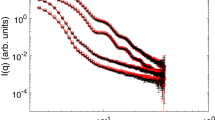
The colloidal sol–gel transition based on zirconyl nitrate solution systems is investigated in this work. The different steps occurring in the transition have been identified by coupling small angle X-ray scattering with Raman spectroscopy and rheology measurements. The effect of the experimental conditions, such as the zirconium precursor concentration and pH, on the transition is studied. The precise mechanisms involved during the transition are based on a detailed understanding of the nanostructure of these systems. In particular, the dissolution of the zirconium salt leads to the formation of cyclic tetramers that self-organize into a cylindrical shape. We clearly demonstrate that increasing the pH induces a strong attractive interaction between the cylinders, giving rise to a mass fractal dimension. For each system, two characteristic pH values have been determined via rheological measurements analysis, where gelation is notably slow below the first pH value and precipitation occurs above the second one. The complete description of the quaternary system (zirconyl nitrate + acetylacetone + ammonia + water) is an efficient formulation guide for the further combination with a templating route leading to structured Zr-based materials.










Similar content being viewed by others
References
Livage J, Henry M, Sanchez C (1988) Sol-gel chemistry of transition-metal oxides. Prog Solid State Chem 18:259–341
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102:4243–4265
Petkova N, Dlugocz S, Gutzov S (2011) Preparation and optical properties of transparent zirconia sol-gel materials. J Non-Cryst Solids 357:1547–1551
Riello P, Minesso A, Craievich A, Benedetti A (2003) Synchrotron SAXS study of the mechanisms of aggregation of sulfate zirconia sols. J Phys Chem B 107:3390–3399
Stawski TM, Besselink R, Veldhuis SA, Castricum HL, Blank DHA, ten Elshof JE (2012) Time-resolved small angle X-ray scattering study of sol-gel precursor solutions of lead zirconate titanate and zirconia. J Colloid Interface Sci 369:184–192
Lemonnier S, Grandjean S, Robisson AC, Jolivet JP (2010) Synthesis of zirconia sol stabilized by trivalent cations (yttrium and neodymium or americium): a precursor for Am-bearing cubic stabilized zirconia. Dalton Trans 39:2254–2262
Mondal A, Ram S (2004) Monolithic t-ZrO2 nanopowder through a ZrO(OH)(2)(.)xH(2)O polymer precursor. J Am Ceram Soc 87:2187–2194
Bai L, Wyrwalski F, Lamonier JF, Khodakov AY, Monflier E, Ponchel A (2013) Effects of beta-cyclodextrin introduction to zirconia supported-cobalt oxide catalysts: from molecule-ion associations to complete oxidation of formaldehyde. Appl Catal B-Environ 138:381–390
Singhal A, Toth LM, Beaucage G, Lin JS, Peterson J (1997) Growth and structure of zirconium hydrous polymers in aqueous solutions. J Colloid Interface Sci 194:470–481
Geiculescu AC, Rack HJ (2002) X-ray scattering studies of polymeric zirconium species in aqueous xerogels. J Non-Cryst Solids 306:30–41
Chepurna I, Smotraev R, Kanibolotsky V, Strelko V (2011) Colloidal and chemical aspects of nanosized hydrated zirconium dioxide synthesized via a sol-gel process. J Colloid Interface Sci 356:404–411
Ambrosi M, Fratini EL, Canton P, Dankesreiter S, Baglioni P (2012) Bottom–up/top–down synthesis of stable zirconium hydroxide nanophases. J Mater Chem 22:23497–23505
Hu MZC, Zielke JT, Lin JS, Byers CH (1999) Small-angle X-ray scattering studies of early-stage colloid formation by thermohydrolytic polymerization of aqueous zirconyl salt solutions. J Mater Res 14:103–113
Southon PD, Bartlett JR, Woolfrey JL, Ben-Nissan B (2002) Formation and characterization of an aqueous zirconium hydroxide colloid. Chem Mater 14:4313–4319
Clearfield A (1964) Structural aspects of zirconium chemistry. Rev Pure Appl Chem 14:91
Ivanov VK, Kopitsa GP, Baranchikov AY, Sharp M, Pranzas K, Grigoriev SV (2009) Mesostructure, fractal properties and thermal decomposition of hydrous zirconia and hafnia. Rus J Inorg Chem 54:2091–2106
Ivanov VK, Kopitsa GP, Baranchikov AE, Grigor’ev SV, Haramus VM (2010) Evolution of composition and fractal structure of hydrous zirconia xerogels during thermal annealing. Rus J Inorganic Chem 55:155–161
Rosa MAA, Sanhueza CSS, Santilli CV, Pulcinelli SH, Briois V (2008) Stimuli-responsive controlled growth of mono- and bidimensional particles from basic zirconium sulfate hydrosols. J Phys Chem B 112:9006–9012
Jolivet J-P, Henry M, Livage J (2000) Metal oxide chemistry and synthesis: from solution to solid state. Wiley, EDP Sciences edn., Colorado
Alves-Rosa MA, Martins L, Pulcinelli SH, Santilli CV (2013) Design of microstructure of zirconia foams from the emulsion template properties. Soft Matter 9:550–558
Konishi J, Fujita K, Oiwa S, Nakanishi K, Hirao K (2008) Crystalline ZrO2 monoliths with well-defined macropores and mesostructured skeletons prepared by combining the alkoxy-derived sol-gel process accompanied by phase separation and the solvothermal process. Chem Mater 20:2165–2173
Kohlbrecher J, Bressler I, SASfit (2011) version 0.93.3, http://kur.web.psi.ch/sans1/SANSSoft/sasfit.html
Kohlbrecher J (2012) Paul Scherrer Institute
Zemb T, Lindner P (2002) Scattering methods applied to soft condensed matter, North Holland
Brinker CJ, Scherer GW (1990) Sol-gel science : the physics and chemistry of sol-gel processing
Clearfield A, Vaughan PA (1956) The crystal structure of zirconyl chloride octahydrate and zirconyl bromide octahydrate. Acta Crystallogr 9:555–558
Mak TCW (1968) Refinement of crystal structure of zirconyl chloride octahydrate. Can J Chem 46:3491
Peyre V, Spalla O, Belloni L, Nabavi M (1997) Stability of a nanometric zirconia colloidal dispersion under compression: effect of surface complexation by acetylacetone. J Colloid Interface Sci 187:184–200
Wu H, Xie JJ, Lattuada M, Morbidelli M (2005) Scattering structure factor of colloidal gels characterized by static light scattering, small-angle light scattering, and small-angle neutron scattering measurements. Langmuir 21:3291–3295
Wu H, Xie JJ, Morbidelli M (2013) Kinetics of colloidal gelation and scaling of the gelation point. Soft Matter 9:4437–4443
Sacks MD, Sheu RS (1987) Rheological properties of silica sol-gel materials. J Non-Cryst Solids 92:383–396
Job N, Panariello F, Crine M, Pirard J-P, Leonard A (2007) Rheological determination of the sol-gel transition during the aqueous synthesis of resorcinol–formaldehyde resins. Colloids Surf a-Physicochem Eng Asp 293:224–228
Winter HH, Chambon F (1986) Analysis of linear viscoelasticity of a cross-linking polymer at the gel point. J Rheol 30:367–382
Ponton A, Barboux-Doeuff S, Sanchez C (2005) Physico-chemical control of sol-gel transition of titanium alkoxide-based materials studied by rheology. J Non-Cryst Solids 351:45–53
Acknowledgments
Financial supports from CEA-DEN are greatly acknowledged. The authors wish to thank Dr. Causse for interesting discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gossard, A., Toquer, G., Grandjean, S. et al. Coupling between SAXS and Raman spectroscopy applied to the gelation of colloidal zirconium oxy-hydroxide systems. J Sol-Gel Sci Technol 71, 571–579 (2014). https://doi.org/10.1007/s10971-014-3409-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3409-2