Abstract
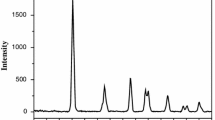
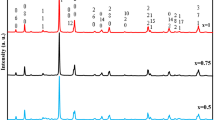
Irradiated semiconductor catalysis in the presence of molecular oxygen can be admitted as an innovative and economical process for the conversion of harmful aromatics to less harmful products. The present work reports the preparation of a co-doped system by sonication assisted sol–gel technique followed by calcination at 500 °C and checks its activity in photo-oxidation reactions. The prepared system was characterized by various physico-chemical techniques such as X-ray diffraction, Raman spectroscopy, IR spectroscopy, TG-DTG, UV-DRS, SEM, and XPS. Thermal stability and structural identity were observed from TG and XRD measurements respectively. On reaction, Tertiary amine appended anthracene and its phenyl substituted derivative in CH3CN yielded Anthraquinone as the major product. Substituted Anthracenemethanamine reacted slowly and a relatively stable intermediate could be isolated at shorter periods of time. The products were separated and purified by column chromatography and the resultant products were characterized thoroughly by 1H NMR, IR spectroscopy and GCMS analysis.












Similar content being viewed by others
References
Kuvarega AT, Krause RWM, Mamba BB (2011) J Phys Chem C 115:22110–22120
Lin YM, Tseng YH, Huang JH, Chao CC, Chen CC, Wang I (2006) Environ Sci Technol 40:1616–1621
Zhao J, Chen C, Ma W (2005) Top Catal 35:269–278
Anpo MJ (2003) Catal 216:233
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269–271
Brezova V, Blazkova A, Karpinsky L, Groskova J, Havlinova B, Jorik V, Ceppan M (1997) J Photochem Photobiol A Chem 109:177–183
Ikeda S, Sugiyama N, Pal B, Marci G, Palmisano L, Noguchi H, Uosaki K, Ohtani B (2001) Phys Chem Chem Phys 3:267–273
Fuerte A, Hernandez-Alonso MD. Maira AJ, Martinez-Arias A, Fernandez-Garcia M, Conesa JC, Soria J (2001) Chem Commun 2718–2719
Kuvarega AT, Krause RWM, Mamba BB (2011) J Phys Chem C 115:22110–22120
Liu J, Han R, Zhao Y, Wang H, Lu W, Yu T, Zhang Y (2011) J Phys Chem C 115:4507–4515
Sun X, Liu H, Dong J, Wei J, Zhang Y (2010) Catal Lett 135:219–225
Shi Z, Yao S, Sui C (2011) Catal Sci Technol 1:817–822
Panayotov DA, Burrows SP, Morris JR (2012) J Phys Chem C 116:6623–6635
Vadakkan JJ, Mallia RR, Prathapan S, Rath NP, Unnikrishnan PA (2005) Tetrahedron Lett 46:5919–5922
Song S, Tu JJ, He ZQ, Hong FY, Liu WP, Chen JM (2010) Appl Catal A 378:169–174
Golubovic A, Scepanovic M, Kremenovic A, Askrabic S, Berec V, Dohcevic-Mitrovic Z, Popovic ZV (2009) J Sol–Gel Sci Technol 49:311–319
Ying W, Yan W, Yanling M, Hanming D, Yougkui S, Xian Z, Xiaozhen T (2008) J Phys Chem C 112:6620–6626
Shu Y, Yohei A, Masakazu K, Jinshu W, Qing T, Tsugio S (2005) J Mater Chem 15:674–682
Ye C, Jinlong Z, Feng C, Masakazu A (2007) J Phys Chem C 111:6976–6982
Liu J, Rong H, Zhao Y, Wang H, Lu W, Yu T, Zhang Y (2011) J Phys Chem C 115:4507–4515
Kubelka P, Munk F (1931) Tech Phys 12:593
Choi H, Antoniou MG, Pelaez M, De la Cruz AA, Shoemaker JA, Dionysiou DD (2007) Environ Sci Technol 41:7530–7535
Sakthivel S, Kish H (2003) Chem Phys Chem 4:487–490
Acknowledgments
MKM is indebted to UGC-BSR for the fellowship. Help received from Dr. S. Prathapan (Associate Professor) and Jomon P. Jacob (SRF), Dept. of Applied Chemistry, CUSAT during photooxidation reaction and STIC, CUSAT for characterization is well acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mothi, K.M., Soumya, G., Salim, A.H. et al. Visible light active Ag, N co-doped titania in the photo-oxidation of some 9-(N,N-dimethylaminomethyl)anthracene systems. J Sol-Gel Sci Technol 71, 549–556 (2014). https://doi.org/10.1007/s10971-014-3389-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3389-2