Abstract
A series of mesoporous carbons (MCs) have been obtained through organic–organic self-assembly method by using phloroglucinol–formaldehyde as carbon precursor and a reverse amphiphilic triblock copolymer as a template. Because of its acidity, the phloroglucinol was used as a catalyst itself. Results show that the pore size and structure of MCs were tailored by simply tuning the weight content of formaldehyde while keeping other reactants constant. A cylindrical mesostructure was obtained when the weight content was 1.0, 1.2 and 1.4. Further increasing the weight content to 1.6 or 2.0, a three-dimensional cage-like mesostructure was obtained. Specific surface area and pore volume up to 485 m2/g and 0.78 cm3/g can be reached, respectively. In addition, the pore size can be tuned in the range of 4.9–14.8 nm by changing the content of formaldehyde.
Similar content being viewed by others
1 Introduction
Mesoporous carbons (MCs), owing to their low mass density, tunable pore size, high surface area, high electrical conductivity, high chemical stability and other interesting properties [1], have been used in lots of fields such as storage of hydrogen and methane [2], electrochemical capacitors [3, 4] and lithium ion battery [5], molecular adsorption and separation [6], catalyst support [7], etc. Conventionally, MCs were successfully prepared through the so-called nanocasting technique [8–11]. Although the nanocasting pathway is a useful tool for the creation of nanostructured carbon materials, the disadvantages (such as the long process and using toxic chemicals to remove the templates) are also very obvious.
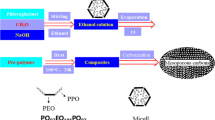
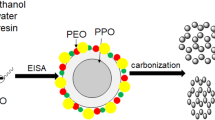
Recently, organic–organic self-assembly was used to prepare MCs with tunable pore size and structure. Many scientists make substantive efforts and have obtained significant achievements [12–21]. For example, Ueyama et al. [18] synthesized MCs by an organic–organic method using resorcinol (R)/formaldehyde (F) and two copolymers ((PO: propyleneoxide; EO: ethyleneoxide) such as F127(EO106PO70EO106) and P123(EO20PO70EO20)) under basic condition, and the pore size could be tuned from 4.4 to 6.8 nm by changing the ratio of R/F or using mixed triblock copolymers. Yuan et al. [19] prepared MCs with ultra-large pore size with the use of triblock copolymers and phloroglucinol/formaldehyde polymer as filler using 37 % HCl as catalyst, and the pore size of MCs could be tailored from 11.5 to 14.7 nm when using P123 as template with the decane as a swelling agent. Furthermore, the MCs exhibit large pore at 19.2 nm with F127 was used as a template to be a substitute for P123. Hayashi et al. [15] also synthesized MCs with pore size from 7.0 to 12.5 nm using different ratios of R/F to F127 and polymerization time. The common drawback in all the above mentioned studies is the need of an acid or basic catalyst due to the low reactivity of phenol or resorcinol with formaldehyde, what will limit their potential application. Liu et al. [20] even pointed out that the concentration of acid and the reactivity of phenols are two key factors which determine the polymerization rate of phenolic resins. It is well-known the phloroglucinol has three hydroxyl groups [22–24], so herein we try to utilize the acidity of phloroglucinol itself to catalyze the reaction with formaldehyde producing resin with more hydroxyl groups which can facilitate a higher degree of cross-linking network. Furthermore, lots of works have been done on the synthesis of MCs with tunable pore size and structure by using mixed copolymers or a pore expanding agent. However, it is still difficult to tailor the pore size of MCs by an individual copolymer template without the addition of small organic molecules such as trimethylbenzene or long-chain n-alkanes as a swelling agent. In this work, we report the direct preparation of MCs using phloroglucinol–formaldehyde composites as carbon precursor and reverse amphiphilic triblock copolymer PO97EO186PO97 as template through soft-template method as shown in Fig. 1.
2 Chemicals and experimental
PO97-EO186-PO97 (MW = 19,500, purchased from Nanjing Co. Limit. Chem. Com). Phloroglucinol, (C6O3H6; 99 %), formaldehyde (HCHO; 37 %), purchased Tianjin Co. Limit. Chem. Com.
Typically, PO97-EO186-PO97 (5 g) was dissolved in ethanol (24 g) under magnetic stirring at 30 °C. Then, the resin solution was synthesized as follows, 3 g of phloroglucinol were dissolved in ethanol (24 g), the pH of solution was about 5–6, followed by addition of a certain amount of 37 % formaldehyde and 8 g of ethanol with stirring at 30 °C for 2 h. Subsequently, the resin solution was added into the ethanol solution containing PO97-EO186-PO97, and stirred for 2 h to form a homogeneous solution. A brown film was obtained by pouring the solution into a dish and allowing the ethanol to evaporate at room temperature for about 24 h, then heating in an oven at 100 °C for 24 h. The removal of template in this manuscript is by direct carbonization at 700 °C just like the references [14, 25] because the template remains only negligible 3.1 % residue at 400 °C. MCs were obtained by direct carbonization of the as synthesized samples at 700 °C for 3 h at a heating rate of 1 Kmin−1 under N2. (here the samples can be denoted as MCs-X, where X is the weight of the formaldehyde, X = 1.0, 1.2, 1.4 1.6 and 2.0, respectively).
Nitrogen adsorption–desorption isotherms were performed at 77 K on a Micromeritics ASAP-2000 volumetric adsorption system. The specific surface area was calculated from the adsorption data in the relative pressure interval from 0.05 to 0.35 using the Brunauer-Emmett-Teller (BET) method. The pore size distribution curve was gained from adsorption branch by using Barrett-Joyner-Halenda (BJH) method. The total pore volume (Vtotal) was calculated at the relative pressure of 0.99. The micropore volume (Vmicro) was determined by t-plot model, and the mesopore volume (Vmeso) was calculated by the difference of Vtotal and Vmicro. Small angle X-ray scattering (SAXS) recorded using an imaging plate with X-ray wavelength of λ = 1.54Å at beam line 1W2A at the Beijing Synchrotron Radiation station. Transmission electron microscopy (TEM, Hitachi operated at 200 kV).
3 Results and discussion
The SAXS patterns of MCs are shown in Fig. 2. It can be found that all samples, excepting MCs-1.2, do not show well resolved peaks at low angles, indicating the disordered pore structure of samples. It is noticeable that the MCs-1.2 gives a small and weak peak in low angle area (as exhibit in the inset of Fig. 2), suggesting that MCs-1.2 present, in some extent, an ordered mesoporous structure [26].
The N2 adsorption/desorption isotherms of the typical samples are shown in Fig. 3a, all samples demonstrate mesoporous character according to the IUPAC classification. It is interesting when the content of formaldehyde varies, so does the shape of the hysteresis loops in MCs. For example, the MCs-1.0, 1.2, 1.4 exhibit a characteristic similar type-IV curve with an obvious H1 hysteresis loop with leap start-points at relative pressures of P/P0 = 0.65, which is typical of uniformly sized mesoporous structure consisting of very large mesopores with an open cylindrical pore geometry [27, 28]. The steep capillary condensation steps indicate that corresponding pore size distributions are narrow as shown in Fig. 3b.
As the content of formaldehyde increases to 1.6 or 2.0, both samples exhibit a characteristic type-IV curve with an H2 hysteresis loops at about relative pressure (P/P0) between 0.4 and 0.8 as demonstrated in Fig. 3a, which implies smaller mesopore size and cage-like type structure [28, 29]. Accordingly, the MC-1.0 has the biggest volume 0.78 cm3/g and MC-2.0 has the smallest volume 0.28 cm3/g as shown in Table 1.
The pore size distributions of MC-1.0, MC-1.2, MC-1.4, MC-1.6 and MC-2.0 are as shown in Fig. 3b. Apparently, it can be found there is a decreasing trend in the pore size distribution with the amount of formaldehyde increasing. For example, the pore size of MCs decreases from 14.8 to 4.9 nm with the content of formaldehyde increasing from 1.0 to 2.0 as shown in Fig. 4. Furthermore, the pore volume also has the similar tendency with the variation of formaldehyde. The mentioned changes in the textural parameters are similar to those observed under basic NaOH condition [25], further confirming our model to tune the pore size and structure.
Figure 5 shows the typical TEM images of MCs. It can be observed that MC-1.2 presents a hexagonal arrangement as demonstrated in Fig. 5a, which is consistent with SAXS result. With the formaldehyde content increasing, the pore structure has transformed into cagelike or ink bottle pore as indicated by the TEM image of MC-2.0 in Fig. 5b. These above results can be explained as follows: the different content of formaldehyde plays a crucial role in the process of formaldehyde/phloroglucinol composite, which further affects the interaction with surfactant through hydrogen bond. The formation of unusual lyotropic phase from reverse triblock copolymer as well as the hydrogen-bonding interaction may also play an important role in the formation of mesoporous structure.
4 Conclusion
In conclusion, MCs with controllable pore size and structure were fabricated using an easy organic–organic process without any acid or basic catalyst. Both the final mesopore size and the mesostructure of MCs can be controlled by changing the amount of formaldehyde. Thus, by increasing formaldehyde ratio, the mesopore size can be tuned from 14.8 to 4.9 nm and the mesostructure symmetry can be changed from hexagonal to cubic. The amount of formaldehyde determines the degree of polymerization, which in turn, determines the interaction resin-Pluronic and the correct replication of the soft-template without a collapse.
References
Liang Y, Fu R, Wu D (2013) ACS Nano 7:1748–1754
Chathoth SM, Mamontov E, Melnichenko YB, Zamponi M (2010) Microporous Mesoporous Mater 132:148–153
Zhao X, Zhang Q, Zhang B, Chen C-M, Wang A, Zhang T, Su DS (2012) J Mater Chem 22:4963–4969
Chen S, Shen W, Zhang S (2011) J Sol Gel Sci Technol 60:131–136
Fang Y, Lv Y, Che R, Wu H, Zhang X, Gu D, Zheng G, Zhao D (2013) J Am Chem Soc 135:1524–1530
Horikawa T, Sakao N, Do DD (2013) Carbon 56:183–192
Xiang D, Yin L (2012) J Mater Chem 22:9584–9593
Jaroniec M, Choma J, Gorka J, Zawislak A (2008) Chem Mater 20:7
Su B-L, Vantomme AL, Surahy L, Pirard R, Pirard J-P (2007) Chem Mater 19:3325–3333
Guo L, Cui X, Li Y, He Q, Zhang L, Bu W, Shi J (2009) Chem Asian J 4:1480–1485
Kim Y, Cho CY, Kang JH, Cho YS, Moon JH (2012) Langmuir ACS J Surf Colloids 28:10543–10550
Liu L, Wang F-Y, Shao G-S, Ma T-Y, Yuan Z-Y (2010) Carbon 48:2660–2664
Sun G, Li K, Xie L, Wang J, Li Y (2012) Microporous Mesoporous Mater 151:282–286
Li P, Song Y, Lin Q, Shi J, Liu L, He L, Ye H, Guo Q (2012) Microporous Mesoporous Mater 159:81–86
Suryavanshi UB, Ijima T, Hayashi A, Hayashi Y, Tanemura M (2011) Chem Commun 47:10758–10760
Ding S, Zheng S, Xie M, Peng L, Guo X, Ding W (2011) Microporous Mesoporous Mater 142:609–613
Yoshimune M, Yamamoto T, Nakaiwa M, Haraya K (2008) Carbon 46:1031–1036
Jin J, Nishiyama N, Egashira Y, Ueyama K (2009) Microporous Mesoporous Mater 118:218–223
Liu L, Wang F-Y, Shao G-S, Ma T-Y, Yuan Z-Y (2010) Carbon 48:2660–2663
Liu C, Li L, Song H, Chen X (2007) Chem Commun 43:757–759
Gutiérrez MC, Picó F, Rubio F, Manuel Amarilla J, Javier Palomares F, Ferrer ML, del Monte F, Rojo JM (2009) J Mater Chem 19:1236–1240
Jirglová H, Pérez-Cadenas AF, Maldonado-Hódar FJ (2009) Langmuir ACS J Surf Colloids 25:2461–2466
Mayes RT, Tsouris C, Kiggans JO Jr, Mahurin SM, DePaoli DW, Dai S (2010) J Mater Chem 20:8674–8678
Liang C, Dai S (2006) J Am Chem Soc 126:5316–5317
Li P, Song Y, Guo Q, Shi J, Liu L (2011) Mater Lett 65:2130–2132
Liu D, Lei J-H, Guo L-P, Qu D, Li Y, Su B-L (2012) Carbon 50:476–487
Ustinov EA, Do DD, Jaroniec M (2005) Appl Surf Sci 252:1013–1028
Górka J, Fenning C, Jaroniec M (2009) Colloids Surf A 352:113–117
Enterría M, Suárez-García F, Martínez-Alonso A, Tascón JMD (2013) Carbon 54:365–377
Acknowledgments
This research was supported by the financial support of Fund of National Nature Science Foundation of China (NO.50602046), ICC CAS Fund for distinguished Young Scientist, State Education Ministry and Natural Science Foundation of Shanxi Province (NO.2007011075). Greatly thanks Prof. Zhi-hong Li and Prof Zhong-hua Wu from institute of High Energy and Physics for SAXS measurements assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, P., Song, Y., Tang, Z. et al. Self-catalyzed synthesis of mesoporous carbons with tunable pore size and structure by soft-templating method. J Sol-Gel Sci Technol 69, 47–51 (2014). https://doi.org/10.1007/s10971-013-3183-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3183-6