Abstract
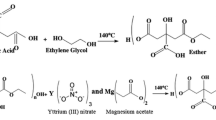
We report the synthesis of nanocrystalline phase-pure YCrO3 powders by a poly acrylic acid (PAA) assisted sol–gel process at a comparatively low calcination temperature of 600 °C. The role of PAA in the powder processing was investigated systemically using Fourier transform infrared (FTIR) spectroscopy, differential thermal and thermogravimetric analyzer (DT/TGA), X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). PAA is found to have the desirable property of forming stable complexes with the cations, Y3+ and Cr3+ at a low pH. FTIR results demonstrated that in the gel-precursor, the carboxylate groups of PAA bond to Y3+ and Cr3+ in a monodentate and a bridging bidentate configuration, respectively, owing to the properties of Cr3+ giving the best correlation with PAA. The obtained particles have capsule-like morphology with a mean diameter of 40 nm. It is presumed that this morphology is due to the extended-chain configuration of PAA in the aqueous solution. The method showed a good control over particle size, morphology, chemical homogeneity, stoichiometry and agglomeration of the powders.









Similar content being viewed by others
References
Kleemann W, Borisov P (2008) In: Luk’yanchuk IA, Mezzane D (eds) Smart materials for energy, communications and security, 1st edn. Springer, Netherlands
Scott JF (2007) Nat Mater 6:256–257
Ghosez P, Triscone JM (2011) Nat Mater 10:269–270
Mostovoy M (2010) Nat Mater 9:188–190
Ramesh R (2009) Nature 461:1218–1219
Martin LW, Crane SP, Chu YH, Holcomb MB, Gajek M, Huijben M, Yang CH, Balke N, Ramesh R (2008) J Phys Condens Matter 20:434220.1–434220.13
Serrao CR, Kundu AK, Krupanidhi SB, Waghmare UV, Rao CNR (2005) Phys Rev B 72:220101.1–220101.4
Tofield BC, Fender BEF (1970) J Phys Chem Solids 31:2741–2749
Ramesha K, Llobet A, Proffen Th, Serrao CR, Rao CNR (2007) J Phys Condens Matter 19:102202.1–102202.8
Keith ML, Roy R (1954) Am Mineral 39:1–23
Weber WJ, Griffin CW, Bates JL (1987) J Am Ceram Soc 70:265–270
Looby JT, Katz L (1954) J Am Chem Soc 76:6029–6030
Tachiwaki T, Kunifusa Y, Yoshinaka M, Hirota K, Yamaguchi O (2001) Int J Inorg Mater 3:107–111
Bedekar V, Shukla R, Tyagi AK (2007) Nanotechnology 18:155706.1–155706.6
Sardar K, Lees MR, Kashtiban RJ, Sloan J, Walton RI (2011) Chem Mater 23:48–56
Zheng M, Gu M, Jin Y, Jin G (2001) Mater Res Bull 36:853–859
Rao BP, Caltun OF, Kim C (2008) J Optoelectron Adv Mater 10:1885–1888
Chen DH, He XR (2001) Mater Res Bull 36:1369–1377
Wang SR, Tseng WJ (2009) J Nanopart Res 11:947–953
Saha SK, Pathak A, Pramanik P (1995) J Mater Sci Lett 14:35–37
Sun YK, Oh IH, Kim KY (1997) J Mater Chem 7:1481–1485
Sun YK, Oh IH, Kim KY (1997) Ind Eng Chem Res 36:4839–4846
Pearson RG (1968) J Chem Educ 45:581–587
Pearson RG (1988) Inorg Chem 27:734–740
Lessing PA (1989) Am Ceram Soc Bull 68:1002–1007
Kirwan LJ, Fawell PD, Bronswijk WV (2003) Langmuir 19:5802–5807
Dong J, Ozaki Y, Nakashima K (1997) Macromolecules 30:1111–1117
Chen HJ, Jian PC, Chen JH, Wang L, Chiu WY (2007) Ceram Int 33:643–653
Wang L, Zhang Y, Zhu Y (2010) Nano Res 3:317–325
Dubinsky S, Grader GS, Shter GE, Silverstein MS (2004) Polym Degrad Stab 86:171–178
Deacon GB, Phillips RJ (1980) Coord Chem Rev 33:227–250
Nara M, Torri H, Tasumi M (1996) J Phys Chem 100:19812–19817
Nair SR, Purohit RD, Tyagi AK, Sinha PK, Sharma BP (2008) Mater Res Bull 43:1573–1582
Suryanarayana C, Norton MG (1998) X-ray diffraction: a practical approach. Plenum Press, New York
Patterson AL (1939) Phys Rev 56:978–982
Duran A, Arevalo-Lopez AM, Castillo-Martinez E, Garcia-Guaderrama M, Moran E, Cruz MP, Fernandez F, Alario-Franco MA (2010) J Solid State Chem 183:1863–1871
Patil DS, Venkatramani N, Rohatgi VK (1988) J Mater Sci Lett 7:413–414
Doyle WP, Eddy P (1967) Spectrochim Acta, Part A 23:1903–1907
Acknowledgments
We thank Mr. Hideo Nishioka of JEOL Limited, Japan for providing the TEM support. Financial assistances from UGC-Govt. of India through SAP and DST-Govt. of India through FIST Program are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnan, S., Kalarikkal, N. Synthesis of YCrO3 nanoparticles through PAA assisted sol–gel route. J Sol-Gel Sci Technol 66, 6–14 (2013). https://doi.org/10.1007/s10971-013-2959-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-2959-z