Abstract
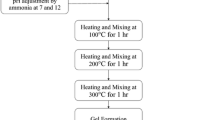
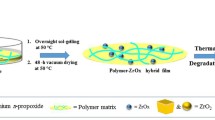
Zirconium oxide is a ceramic material widely studied due to its mechanical and electrical properties that can be improved with the use of carbon nanotubes (CNTs) as reinforcement. The synthesis of CNT/zirconia composites by sol–gel method is still very scarce, due to the hydrophobic nature of the CNTs, being their dispersion in aqueous medium an intrinsic difficulty to the synthesis. In this work, we present a sol–gel synthesis for MWCNTs/zirconia composites, where two kinds of surfactants, sodium and ammonium stearates dissolved in water (1 g/100 mL), were used as dispersant agents for multiwall carbon nanotubes (MWCNTs). They are cheap and easy to prepare, and were very effective in dispersing the MWCNTs. Different quantities of MWCNTs (up to 5 wt%) were added in the solution of stearate/water and this solution with the highly dispersed MWCNTs was added to the zirconia sol–gel, producing composites of MWCNTs/zirconia with different concentrations of MWCNTs. All the powders were heat treated at 300 and 500 °C and the powder characterization was performed by transmission electron microscopy (TEM), X-ray diffraction (XRD), thermogravimetric analysis (TGA) and infrared spectroscopy (FTIR). The composite MWCNTs/zirconia remained amorphous at 300 °C and presented a tetragonal phase at 500 °C with an average grain size of about 20 ± 3 nm, determined by the Scherrer equation from the XRD patterns. For these crystalline samples, TEM images suggest a more effective interaction between MWCNTs with ZrO2 matrix, where it can be observed that the carbon nanotubes are fully coated by the matrix.










Similar content being viewed by others
References
Cho J, Boccaccini AR, Schaffer MSP (2009) J Mater Sci 44:1934–1951
Arsent’ev MY, Tikhonov PA, Kalinina MV, Andreeva NS (2010) Glass Phys Chem 36(4): 478–483
Díaz-Parralejo A, Ortiz AL, Caruso R (2010) Ceram Int 36:2281–2286
Subbarao EC (1981) In: Sience and technology of zirconia. The American Ceramic Society, Columbus
Vollath D, Fischer F, Hagelstein M, Szabó DJ (2006) Nanopart Res 8:1003–1016
Santos V, Zeni M, Bergmann CP, Hohemberger JM (2008) Rev Adv Mater Sci 17:62–70
Brincker CJ, Scherer GW (1990) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, San Diego
Corriu R, Trong Anh N (2009) Molecular chemistry of sol-gel derived nanomaterials. Wiley, New York
Andrulevicius M, Tamulevicius S, Gnatyuk Y, Vityuk N, Smirnova N, Eremenko A (2008) Mat Sci 14(1):08–14
Zhang Y, Pan L, Gao C, Wang Y, Zhao Y (2010) J Sol-Gel Sci Technol 56:27–32
Saito S, Zettl A (2008) Carbon nanotubes quantum cylinders of graphene. Elsevier, Amsterdam
Pradeep T (2007) Nano the essentials: understanding nanoscience and nanotechnology. McGraw-Hill, New York
Hu Y, Shenderova OA, Hu Z, Padgett CW, Brenner DW (2006) Rep Progr Phys 69:1847–1895
de Andrade MJ, Lima MD, Bergmann CP, Ramminger GO, Balzaretti NM, Costa TMH, Gallas MR (2008) Nanotechnol 19:265607
Ji Y, Huang YY, Tajbakhsh AR, Terentjev EM (2009) Langmuir 25(20):12325–12331
Vaisman L, Wagner HD, Marom G (2006) Adv Colloid Interface Sci 128–130:37–46
Silva PR, Almeida VO, Machado GB, Benvenutti EV, Costa TMH, Gallas MR (2012) Langmuir 28:1447–1452
Cappele HA, Britcher LG, Morris GE (2003) J Colloid Interface Sci 268:293–300
Huang W, Yang J, Meng X, Cheng Y, Wang C, Zou B, Khana Z, Wang Z, Cao X (2011) Chem Eng J 168:1360–1368
Parralejo AD, García AM, González JS, Díez MAD, Correa EMC (2011) J Non-Cryst Solids 357: 1090–1095
Agoudjila N, Kermadia S, Larbotb A (2008) Desalination 223:417–424
Sumana G, Das M, Srivastava S, Malhotra BD (2010) Thin Solid Films 519:1187–1191
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almeida, V.O., Balzaretti, N.M., Costa, T.M.H. et al. Surfactants for CNTs dispersion in zirconia-based ceramic matrix by sol–gel method. J Sol-Gel Sci Technol 65, 143–149 (2013). https://doi.org/10.1007/s10971-012-2918-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2918-0