Abstract
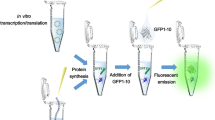
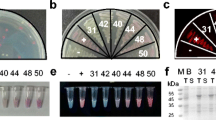
We have improved our green fluorescent protein (GFP) folding reporter technology [Waldo et al., (1999) Nat. Biotechnol. 17, 691–695] to evolve recalcitrant proteins from Mycobacterium tuberculosis. The target protein is inserted into the scaffolding of the GFP, eliminating false-positive artifacts caused by expression of truncated protein variants from internal cryptic ribosome binding sites in the target RNA. In parallel, we have developed a new quantitative fluorescent protein tagging and detection system based on micro-domains of GFP. This split-GFP system, which works both in vivo and in vitro, is amenable to high-throughput assays of protein expression and solubility [Cabantous et al., (2005) Nat. Biotechnol. 23, 102–107]. Together, the GFP folding reporter and split-GFP technologies offer a comprehensive system for manipulating and improving protein folding and solubility.
Similar content being viewed by others
Abbreviations
- AnTET:
-
anhydrotetracycline
- GFP:
-
green fluorescent protein
- IPTG:
-
isopropyl thiogalactoside
- MBP:
-
maltose-binding protein
- NaCl:
-
sodium chloride
- SUMO:
-
small ubiquitin-like modifiers
References
G. Georgiou P. Valax (1996) Curr. Opin. Biotechnol. 7 190–197 Occurrence Handle10.1016/S0958-1669(96)80012-7 Occurrence Handle8791338
R.C. Hockney (1994) Trends Biotechnol. 12 456–463 Occurrence Handle10.1016/0167-7799(94)90021-3 Occurrence Handle7765545
K. Yokoyama Y. Kikuchi H. Yasueda (1998) Biosci. Biotechnol. Biochem. 62 1205–1210 Occurrence Handle10.1271/bbb.62.1205 Occurrence Handle9692205
K.H. Lee H.S. Kim H.S. Jeong Y.S. Lee (2002) Biochem. Biophys. Res. Commun. 298 216–224 Occurrence Handle10.1016/S0006-291X(02)02423-3 Occurrence Handle12387818
Y. Chen J. Song S.F. Sui D.N. Wang (2003) Protein Exp. Purif. 32 221–231 Occurrence Handle10.1016/S1046-5928(03)00233-X
F. Baneyx J.L. Palumbo (2003) Methods Mol. Biol. 205 171–197 Occurrence Handle12491886
J.D. Fox R.B. Kapust D.S. Waugh (2001) Protein Sci. 10 622–630 Occurrence Handle10.1110/ps.45201 Occurrence Handle11344330
J.D. Fox D.S. Waugh (2003) Methods Mol. Biol. 205 99–117 Occurrence Handle12491882
M.P. Malakhov R. Michael O.A.M. Mattern M. Drinker D. Stephen W.T.R. Butt* (2004) J. Struct. Func. Genom 5 75–86 Occurrence Handle10.1023/B:JSFG.0000029237.70316.52
N. Armstrong A. Lencastre Particlede E. Gouaux (1999) Protein Sci. 8 1475–1483 Occurrence Handle10422836
L. Tresaugues B. Collinet P. Minard G. Henckes R. Aufrere K. Blondeau D. Liger Z. Cong-Zhao J. Janin H. Tilbeurgh ParticleVan S. Quevillon-Cheruel (2004) J. Struct. Func. Genom. 5 195–204 Occurrence Handle10.1023/B:JSFG.0000029017.46332.e3
E.A. Woestenenk M. Hammarstrom T. Hard H. Berglund (2003) Anal. Biochem. 318 71–79 Occurrence Handle10.1016/S0003-2697(03)00162-3 Occurrence Handle12782033
L.M. Galvao-Botton A.M. Katsuyama C.R. Guzzo F.C. Almeida C.S. Farah A.P. Valente (2003) FEBS Lett. 552 207–213 Occurrence Handle10.1016/S0014-5793(03)00926-8 Occurrence Handle14527688
W.P. Stemmer (1994) Proc. Natl. Acad. Sci. USA 91 10747–10751 Occurrence Handle7938023
G.S. Waldo B.M. Standish J. Berendzen T.C. Terwilliger (1999) Nat. Biotechnol. 17 691–695 Occurrence Handle10.1038/10904 Occurrence Handle10404163
J.K. Yang H.J. Yoon H.J. Ahn B.I. Lee S.H. Cho G.S. Waldo M.S. Park S.W. Suh (2002) Acta Crystallogr. D Biol. Crystallogr. 58 303–305 Occurrence Handle10.1107/S0907444901018789 Occurrence Handle11807257
C. Wurth N.K. Guimard M.H. Hecht (2002) J. Mol. Biol. 319 1279–1290 Occurrence Handle10.1016/S0022-2836(02)00399-6 Occurrence Handle12079364
J.D. Pedelacq E. Piltch E.C. Liong J. Berendzen C.Y. Kim B.S. Rho M.S. Park T.C. Terwilliger G.S. Waldo (2002) Nat. Biotechnol. 20 927–932 Occurrence Handle10.1038/nbt732 Occurrence Handle12205510
J.K. Yang M.S. Park G.S. Waldo S.W. Suh (2003) Proc. Natl. Acad. Sci. USA 100 455–460 Occurrence Handle10.1073/pnas.0137017100 Occurrence Handle12524453
S. Cabantous T.C. Terwilliger G.S. Waldo (2005) Nat. Biotechnol. 23 102–107 Occurrence Handle10.1038/nbt1044 Occurrence Handle15580262
F.W. Studier (1991) J. Mol. Biol. 219 37–44 Occurrence Handle10.1016/0022-2836(91)90855-Z Occurrence Handle2023259
R. Lutz H. Bujard (1997) Nucleic Acids Res. 25 1203–1210 Occurrence Handle10.1093/nar/25.6.1203 Occurrence Handle9092630
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabantous, S., Pédelacq, JD., Mark, B. et al. Recent Advances in GFP Folding Reporter and Split-GFP Solubility Reporter Technologies. Application to Improving the Folding and Solubility of Recalcitrant Proteins from Mycobacterium tuberculosis. J Struct Funct Genomics 6, 113–119 (2005). https://doi.org/10.1007/s10969-005-5247-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10969-005-5247-5