Abstract
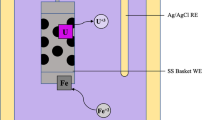
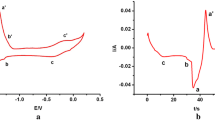
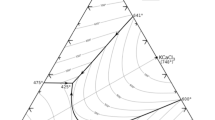
Electrochemical behaviors of U4+ in LiCl–KCl–UF4 eutectic and deposition of U metal were investigated. It was found that the presence of F− has influence on the diffusion of U3+ and U4+ as comparing to data obtained in pure chloride molten salts. Electrochemical deposition of U was carried out by using pulse current electrolysis. Characterization results indicate that U metal was obtained at the cathode, implying U metal can be directly deposited from LiCl–KCl–UF4 eutectic in this case and the extractive ratio is calculated to be 98%. Our results demonstrate feasible separation of U from LiCl–KCl–UF4 molten salt by electrochemical method.





Similar content being viewed by others
References
Laidler JJ (1993) Pyrochemical recovery of actinides; ANL-CMT-CP-78937
Li X, Sofi T, Johnson TA, et al (1999) Experimental observations on electrorefining spent nuclear fuel in molten LiC1–KCl/liquid cadmium system, INL-NT-CP-98226
Laidler JJ, Miller WE, Johnson TR, et al (1992) IFR fuel cycle-pyroprocess development, ANL-CMT-CP-77849
Laidler JJ (1994) Pyrochemical processing of DOE spent nuclear fuel; ANL-CMT-CP-84355
Eddie CG, William EM (1994) Electrorefining “N” reactor fuel’ANL-CMT-CP-84354
Kumar S, Todd A, Mark A et al (2012) Thermal properties of LiCl–KCl molten salt for nuclear waste separation; University of Wisconsin, Project No. 09-780
Kuznetsov SA, Gaune-Escard M (2009) Electrochemical transient techniques for study of the electrochemistry and thermodynamics of nuclear materials in molten salts. J Nucl Mater 389:108–114. doi:10.1016/j.jnucmat.2009.01.015
Sakamurar Y, Hijikata T, Kinoshita K et al (1998) Measurement of standard potentials of actinides (U, Np, Pu, Am) in LiCl–KCl eutectic salt and separation of actinides from rare earths by electrorefining. J Alloy Compd 271–273:592–596. doi:10.1016/S0925-8388(98)00166-2
Shirai O, Iwai T, Suzuki Y et al (1998) Electrochemical behavior of actinide ions in LiCI–KCl eutectic melts. J Alloy Compd 271–273:685–688. doi:10.1016/S0925-8388(98)00187-x
Gao FX, Wang CS, Liu LS et al (2009) Electrode processes of uranium ions and electro deposition of uranium in molten LiCl–KCl. J Radioanal Nucl Chem 280:207–218. doi:10.1007/s10967-008-7417-y
Wang XB, Huang W, Jiang F et al (2016) Electrochemical behavior of Th(IV) and its electrodeposition from ThF4–LiCl–KCl melt. Electrochim Acta 196:286–293. doi:10.1016/j.electacta.2016.02.184
Jenkins HW, Mamantov G, Manning DL (1968) E.M.F. measurements on the nickel-nickel(II) couple in molten fluorides. J Electroanal Chem 19:385–389. doi:10.1016/S0022-0728(68)80101-9
Gao P, Jin XB, Wang D (2005) A quartz sealed Ag/AgCl reference electrode for CaCl2 based molten salts. J Electroanal Chem 579:321–328. doi:10.1016/j.jelechem.2005.03.004
Heinze, J. (1984) Cyclic voltammetry-”electrochemical spectroscopy”. Angew Chem Int Edition, 23: 831–847. doi: 10.1002/anie.198408313
Bard AJ (1980) Electrochemical methods fundamentals and applications, 2nd edn. New York, Wiley
Caravaca C, de Cordoba G, Tomas MJ et al (2007) Electrochemical behavior of gadolinium ion in molten LiCl–KCl eutectic. J Nucl Mater 360:25–31. doi:10.1016/j.jnucmat.2006.08.009
Yang YS, Zhang ML (2014) Selective extraction of gadolinium from Sm2O3 and Gd2O3 mixtures in a single step assisted by MgCl2 in LiCl–KCl melts. J Solid State Electrochem 18:843–850. doi:10.1007/s10008-013-2333-7
Lee YJ, Lee TH, Nersisyan HH et al (2015) Characterization of Ta-W alloy film deposition by molten salt Multi-Anode Reactive alloy Coating (MARC) method. Int J Refract Metals Hard Mater 53:23–31. doi:10.1016/j.ijrmhm.2015.04.022
Tetsuya T, Yuichi I, Takashi A et al (2012) Al-W alloy deposition from lewis acidic room-temperature chloroaluminate ionic liquid. ECS Trans 50:239–250. doi:10.1149/05011.0239ecst
Shannon RD (1976) Revised effective ionic radius and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767. doi:10.1107/S0567739476001551
Smirnov MV (1973) Electrode potentials in molten chlorides. Nauka, Moscow
Patrick M, David B, Rudy K et al (2005) Electrochemistry of uranium in molten LiCl–KCl eutectic. J Electrochem Soc 152:A1109–A1115. doi:10.1149/1.1901083
Acknowledegments
This work was supported by “Strategic Priority Research Program” Chinese Academy of Sciences (Grant No. XDA02030000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, T., Huang, W., Zheng, H. et al. Investigating the influence of F− on U4+ in molten LiCl–KCl–UF4 system and electro-deposition of U. J Radioanal Nucl Chem 312, 479–485 (2017). https://doi.org/10.1007/s10967-017-5242-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5242-x