Abstract
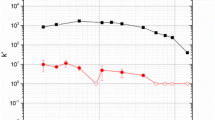
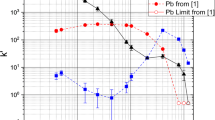
A crown-ether-based extraction chromatography resin, Eichrom Pb resin, was characterized for separations of flerovium (Fl) homologs, specifically Pb and Sn. The batch uptake of Pb(II) and Sn(IV) radionuclides was determined from an HCl matrix. Both Pb(II) and Sn(IV) are strongly retained on the resin at different HCl concentrations. The affinity for Pb(II) decreases with increasing HCl concentration while Sn(IV) uptake increases. Extraction kinetics for Pb(II) and Sn(IV) were examined and show suitable uptake on the second time scale. Separation methods for the isolation of individual homologs, Pb(II) and Sn(IV), have been established using 2 mL pre-packed vacuum flow Pb resin columns.





Similar content being viewed by others
References
Guillaumont T, Adloff JP, Peneloux A (1989) Kinetic and thermodynamic aspects of tracer-scale and single atom chemistry. Radiochim Acta 46:169–176
Schädel M, Shaughnessy DA (eds) (2014) The chemistry of the super heavy elements. Springer, Heidelberg
YuTs Oganessian, Utyonkov VK (2015) Superheavy nuclei from 48Ca induced reactions. Nucl Phys A 944:62–98
Pershina V (1996) Electronic structure and properties of the transactinides and their compounds. Chem Rev 96:1977–2010
Pyykko P, Desclaux J-P (1979) Relativity and the periodic system of elements. Acc Chem Res 12:276–281
Pershina V (2010) Electronic structures and chemistry of the heaviest elements. In: Leszczynski J, Barysz M, Ishikawa Y (eds) Relativistic methods for Chemists. Springer, Dordrecht, pp 451–520
Pitzer KS (1975) Are elements 112, 114, and 118 relatively inert gases? J Chem Phys 63:1032–1033
Nash CS (2005) Atomic and molecular properties of elements 112, 114 and 118. J Phys Chem A 109:3493–3500
Liu W, van Wüllen C, Han YK, Choi YJ, Lee YS (2001) Spectroscopic constants of Pb and Eka-lead compounds: comparison of different approaches. Adv Quant Chem 39:325–355
Hoffman DC, Lee DM, Pershina V (2006) In: Vertes A, Klencsar Z (eds) The chemistry of the actinide and transactinide elements. Springer, Dordrecht, pp 1652–1752
Pershina V, Anton J, Fricke B (2007) Intermetallic compounds of the heaviest elements and their homologs: the electronic structure and bonding of MM′, where M = Ge, Sn, Pb and element 114, and M′ = Ni, Pd, Pt, Cu, Ag, Au, Sn, Pb, and element 114. J Chem Phys 127:134310
Eichler R, Aksenov NV, Albin YV, Belozerov AV, Bozhikov GA, Chepigin VI, Dmitriev SN, Dressler R, Gäggeler HW, Gorshikov VA, Henderson RA, Johnsen AM, Kenneally JM, Lebedev VY, Malyshev ON, Moody KJ, Oganessian YT, Petrushkin OV, Piguet D, Popeko AG, Rasmussen P, Serov A, Shaughnessy DA, Shishkin SV, Shutov AV, Stoyer MA, Stoyer NJ, Svirikhin AI, Tereshatov EE, Vostokin GK, Wegrzecki M, Wilk PA, Wittwer D, Yeremin AV (2010) Indication for a volatile element 114. Radiochim Acta 98:133–139
Yakushev A, Gates JM, Türler A, Schädel M, Düllmann CE, Eberhardt K, Essel HG, Even J, Forsberg U, Gorshkov A, Graeger R, Gregorich KE, Hartmann W, Herzberg R-D, Heßberger FP, Hild D, Hübner A, Jäger E, Khuyagbaatar J, Kindler B, Kratz JV, Krier J, Kurz N, Lommel B, Niewisch LJ, Nitsche H, Omtvedt JP, Parr E, Qin Z, Rudolph D, Runke J, Schausten B, Schimpf E, Semchenkov A, Steiner J, Thörle-Pospiech P, Uusitalo J, Wegrzecki M, Wiehl N (2014) Superheavy element flerovium (element 114) is a volatile metal. Inorg Chem 53:1624–1629
Yordanov AT, Roundhill MD (1998) Solution extractrion of transition and post-transition heavy and precious metals by chelate and macrocyclic ligands. Coord Chem Rev 170:93–124
Khopkar SM (2008) Analytical chemistry of macrocyclic and supramolecular compounds. Narosa Publishing House Pvt Ltd, New Delhi
Weber E, Toner JL, Goldberg I, Vögtle F, Laidler DA, Stoddart JF, Bartsch RA, Liotta CL (1989) Chapter 4 Crown ethers–complexes and selectivity. Crown Ethers and Analogs. Wiley, Chichester, pp 207–304
Izatt RM, Pawlak K, Bradshaw JS, Bruening RL (1991) Thermodynamic and kinetic data for macrocyclic interactions with cations and anions. Chem Rev 98:1721–2085
Horwitz EP, Dietz ML, Rhoads S, Felinto C, Gale NH, Houghton J (1994) A lead-selective extraction chromatographic resin and its application to the isolation of lead from geological samples. Anal Chim Acta 292:263–273
Seth M, Faegri K, Schwerdtfeger P (1998) The stability of the oxidation state +4 in group 14 compounds from carbon to element 114. Angew Chem Int Ed Engl 37:2493–2496
Nervik WE (1960) The radiochemistry of tin. Subcommittee on radiochemistry, National Acadamy of Sciences-National Research Council, Washington D.C
Gibson WM (1961) The radiochemistry of lead. Subcommittee on Radiochemistry, National Academy of Sciences-National Research Council, Washington D.C
Despotopulos JD (2015) Studies of flerovium and element 115 homologs with macrocyclic extractants (Doctoral dissertation). ProQuest, Ann Arbor 3715057:224
Rieman W, Walton HF (1970) Ion exchange in analytical chemistry. Pergamon, Oxford
Despotopulos JD, Kmak KN, Gharibyan N, Brown TA, Grant PM, Henderson RA, Moody KJ, Tumey SJ, Shaughnessy DA, Sudowe R (2015) Production and isolation of carrier-free homologs of flerovium and element 115 at the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4500-z
National Nuclear Data Center “NNDC”(2013) Brookhaven National Llaboratory, [Online]. Available: http://www.nndc.bnl.gov/. Accessed 16 Dec 2013
Horwitz EP, Bloomquist CA (1972) Preparation, performance, and factors affecting band spreading of high-efficiency extraction chromatographic columns for actinide separations. J Inorg Nucl Chem 34:3851–3871
Johnson JS, Kraus KA (1959) Hydrolitic behavior of metal ions IX. Ultracentrifugation of Sn(IV) in acidic chloride and perchlorate solutions. J Phys Chem 63:440–441
Kulrestha NK, Dey AK, Ghosh S (1955) Untersuchungen über hydratisiertes zinnoxyd. Kolloid-Z 141:106–109
Izatt RM, Haymore BL, Christensen JJ (1972) A stable OH3+–cyclic polyether complex characterised by infrared spectroscopy. J. Chem Soc Chem Comm 23:1308–1309
Junk PC (2001) Structural aspects of oxonium ion/crown ether complexes. Rev Anal Chem 21:93–124
Acknowledgments
The authors would like to thank the CAMS facility staff at LLNL, specifically Scott Tumey, Thomas Brown and Graham Bench for providing beam time and expertise to the production of radionuclides used in this study. This study was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. This work was funded by the Laboratory Directed Research and Development Program at LLNL under project tracking code 11-ERD-011, as well as by the LLNL Livermore Graduate Scholar Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Despotopulos, J.D., Kmak, K.N., Gharibyan, N. et al. Characterization of the homologs of flerovium with crown ether based extraction chromatography resins: studies in hydrochloric acid. J Radioanal Nucl Chem 310, 1201–1207 (2016). https://doi.org/10.1007/s10967-016-4917-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4917-z