Abstract
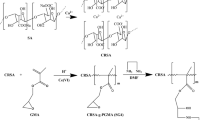
Radiation-induced grafting of acrylic acid onto alginate/chitosan beads was performed in solution at a dose rate of 20.6 Gy/min of Co-60 gamma rays. The effect of absorbed dose on grafting yield was investigated. The characterization of the grafted and un-grafted beads was performed by FTIR spectroscopy and the swelling measurements at different pHs was studied. It is found that as the pH value increases the swelling degree increases up to pH 6 but with further increase in pH value the swelling decreases. Also, it is noticed that the grafting yield increased with increase the irradiation dose. Both un-grafted and grafted alginate/chitosan beads were examined as sorbents for the removal of Pb ions from aqueous solutions. The sorption behavior of the sorbents was examined through pH, and equilibrium measurements. Grafted alginate/chitosan beads presented higher sorption capacity for Pb ions than un-grafted beads.








Similar content being viewed by others
References
Stevens JB (1991) Environ Sci Technol 25:1289
Zaijun L, Jian T, Jiaomai P (2004) Food Control 15:565
Burke DM, Morris MA, Holmes JD (2013) Chemical oxidation of mesoporous carbon foams for lead ion adsorption. Sep Purif Technol 104:150–159
Wang Y, Wang X, Wang X, Liu M, Wu Z, Yang L et al (2013) Adsorption of Pb(II) from aqueous solution to Ni-doped bamboo charcoal. J Ind Eng Chem 19:353–359
Idris A, Ismail NSM, Hassan N, Misran E, Ngomsik A-F (2012) Synthesis of magnetic alginate beads based on maghemite nanoparticles for Pb(II) removal in aqueous solution. J Ind Eng Chem 18:1582–1589
Fan L, Luo C, Sun M, Li X, Qiu H (2013) Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surf B 103:523–529
Rahmani A, Zavvar Mousavi H, Fazli M (2010) Effect of nanostructure alumina on adsorption of heavy metals. Desalination 253:94–100
Dong L, Zhu Z, Ma H, Qiu Y, Zhao J (2010) Simultaneous adsorption of lead and cadmium on MnO2-loaded resin. J Environ Sci 22:225–229
Heidari A, Younesi H, Mehraban Z (2009) Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem Eng J 153:70–79
Lawal OS, Sanni AR, Ajayi IA, Rabiu OO (2010) Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead (II) ions onto the seed husk of Calophyllum inophyllum. J Hazard Mater 177:829–835
O¨nal S, Baysal SH, Ozdemir G, Adebowale Kayode O (2007) Studies on the applicability of alginate-entrapped Chryseomonas luteola TEM 05 for heavy metal biosorption. J Hazard Mater 146:417–420
Unuabonah EI, El-Khaiary MI, Olu-Owolabid BI, Adebowale KO (2012) Chem Eng Res Des 90:1105–1115
Wan Ngah WS, Endud CS, Mayanar R (2002) Removal of copper (II) ions from aqueous solution onto chitosan and crosslinked chitosan beads. React Funct Polym 50:181–190
George B, Pillai VNR, Mathew B (1999) Effect of the nature of the crosslinking agent on the metal-ion complexation characteristics of 4 mol% DVB and NMBA crosslinked polyacrylamide-supported glycines. J Appl Polym Sci 74:3432–3444
Mathew B, Pillai VNR (1993) Polymer-metal complexes of amino functionalized divinylbenzene-crosslinked polyacrylamides. J Polym 34:2650–2658
Kas¸go¨ z H, O¨ zgu¨mu¨ s¸ S, Orbay M (2001) Preparation of modified polyacrylamide hydrogels and application in removal of Cu (II) ion. J Polym 42:7497–7502
Kaşgöz H, Özgümüş S, Orbay M (2003) Modified polyacrylamide hydrogels and their application in removal of heavy metal ions. Polymer 44:1785–1793
Rivas BL, Seguel GV, Geckeler KE (2002) Synthesis, characterization and properties of polychelates of poly (styrene sulfonic acid-co-maleic acid) with Co (II), Cu (II), Ni (II) and Zn (II). J Appl Polym Sci 85:2546–2551
Masri MS, Reuter FW, Friedman M (1974) Binding of metal cations by natural substances. J Appl Polym Sci 18:675–681
Kaminski W, Modrzejewsk Z (1997) Application of chitosan membranes in separation of heavy metal ions. Sep Sci Technol 32(16):2659–2688
Mckay G, Blair HS, Chem AFIJO (1989) Equilibrium studies for the sorption of metal ions onto chitosan. Ind J Chem 28A:356–360
Bajpai SK, Sharma S (2004) Investigation of swelling/degradation behavior of alginate beads crosslinked with Ca2+ and Ba2+ ions. React Funct Polym 59:129–140
Singh V, Sharma AK, Tripathi DN, Sanghi R (2009) Poly (methylmethacrylate) grafted chitosan: an efficient adsorbent for anionic azo dyes. J Hazard Mater 161:955–966
Long Z, Hiroshi M (2009) Hydrogels of dihydroxy propyl chitosan crosslinked with irradiation at paste-like condition. Carbohydr Polym 76(2):314–319
Udayabhaskar P, Iyengar L, Rao AVSP (1990) Hexavalent chromium interaction with chitosan. J Appl Polym Sci 39:739–749
Ng JCY, Cheung WH, Mckay G (2002) Equilibrium studies of the sorption of Cu (II) ions onto chitosan. J. Colloid Interface Sci 255:64–74
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abou El Fadl, F.I. Radiation grafting of ionically crosslinked alginate/chitosan beads with acrylic acid for lead sorption. J Radioanal Nucl Chem 301, 529–535 (2014). https://doi.org/10.1007/s10967-014-3149-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3149-3