Abstract
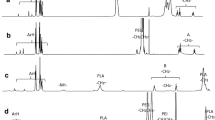
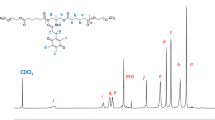
A novel pH-sensitive charge reversal and self-fluorescent polymeric micelle is designed and synthesized successfully. The smart micelle is prepared based on MPEG-polyurethane multi-block copolymer, which is synthesized by polycondensation, with 1, 4-bis (hydroxyethyl) piperazine (HEP) and hydroxyl-sulfamethazine (Hydroxyl-SM) as pH-sensitive molecules and fluorescein isothiocyanate (FITC) as fluorescent segment. The resulting MPEG-polyurethane multi-block copolymer is examined by 1H–NMR, UV-vis spectra, fluorescence spectra and an acid-base titration. Moreover, the diameter, morphology and cytotoxicity of obtained polymer micelle are measured by dynamic light scattering (DLS), transmission electron microscopy (TEM) and MTT assay. The results indicate that the micelle has a small diameter of less than 200 nm and can remain unchanged within two weeks. Zeta-potential measurement shows that the negative charge of micelle can switch into positive charge as the pH value decreasing from 9.0 to 3.0. Subsequently, the fluorescence intensity decreases significantly with the reducing of pH values. The MTT assay shows the low cytotoxicity and good biocompatibility of the MPEG-polyurethane polymeric micelles. Finally, doxorubicin (DOX) is loaded into micelles to detect in vitro release behavior. The drug loaded micelles show a faster release behavior at pH 5.0 than that at pH 7.4. Therefore, the pH-sensitive charge reversal and self-fluorescent micelle can be a potential smart carrier for delivery and controlled release of protein drug.












Similar content being viewed by others
References
Rengaswamy V, Zimmer D, Süss R, Rössler J (2016) RGD liposome-protamine-siRNA (LPR) nanoparticles targeting PAX3-FOXO1 for alveolar rhabdomyosarcoma therapy. J Control Release 235:319–327
Nakamura H, Abu Lila AS, Nishio M, et al (2015) Intra-tumor distribution of PEGylated liposome upon repeated injection: no possession by prior dose. J Control Release 220:406–413
Assanhou AG, Li W, Zhang L, et al (2015) Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials 73:284–295
Dhanya S, Bahadur D, Kundu GC, Srivastava R (2013) Maleic acid incorporated poly-(N-isopropylacrylamide) polymer nanogels for dual-responsive delivery of doxorubicin hydrochloride. Eur Polym J 49:22–32
Huang K, Shi B, Xu W, et al (2015) Reduction-responsive polypeptide nanogel delivers antitumor drug for improved efficacy and safety. Acta Biomater 27:179–193
Llacua A, de Haan BJ, Smink SA, de Vos P (2016) Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes: Extracellar matrix components. J Biomed Mater Res A 104:1788–1796
Ye C, Combs ZA, Calabrese R, et al (2014) Robust microcapsules with controlled permeability from silk fibroin reinforced with graphene oxide. Small 10:5087–5097
Pavlov AM, De Geest BG, Louage B, et al (2013) Magnetically engineered microcapsules as intracellular anchors for remote control over cellular mobility. Adv Mater 25:6945–6950
Liu F, Kozlovskaya V, Medipelli S, et al (2015) Temperature-sensitive Polymersomes for controlled delivery of anticancer drugs. Chem Mater 27:7945–7956
Curcio M, Cirillo G, Vittorio O, et al (2015) Hydrolyzed gelatin-based polymersomes as delivery devices of anticancer drugs. Eur Polym J 67:304–313
Li H, Fu Y, Zhang T, et al (2015) Rational Design of Polymeric Hybrid Micelles with highly tunable properties to co-deliver MicroRNA-34a and Vismodegib for melanoma therapy. Adv Funct Mater 25:7457–7469
Li W, Zheng C, Pan Z, et al (2016) Smart hyaluronidase-actived theranostic micelles for dual-modal imaging guided photodynamic therapy. Biomaterials 101:10–19
Bao Y, Yin M, Hu X, et al (2016) A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J Control Release 235:182–194
Zhang Y, Wang C, Huang Y, et al (2015) Core-crosslinked polymeric micelles with high doxorubicin loading capacity and intracellular pH- and redox-triggered payload release. Eur Polym J 68:104–114
Sun H, Guo B, Cheng R, et al (2009) Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 30:6358–6366
Sun H, Meng F, Cheng R, et al (2014) Reduction-responsive polymeric micelles and vesicles for triggered intracellular drug release. Antioxid Redox Signal 21:755–767
Liu C, Yuan J, Luo X, et al (2014) Folate-decorated and Reduction-sensitive micelles assembled from amphiphilic polymer–Camptothecin conjugates for intracellular drug delivery. Mol Pharm 11:4258–4269
Kataoka K, Harada A, Nagasaki Y (2012) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 64:37–48
Tian H, Tang Z, Zhuang X, et al (2012) Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog Polym Sci 37:237–280
Cai M, Leng M, Lu A, et al (2015) Synthesis of amphiphilic copolymers containing zwitterionic sulfobetaine as pH and redox responsive drug carriers. Colloids Surf B Biointerfaces 126:1–9
Li Y, Heo HJ, Gao GH, et al (2011) Synthesis and characterization of an amphiphilic graft polymer and its potential as a pH-sensitive drug carrier. Polymer 52:3304–3310
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul 41:189–207
Maeda H, Sawa T, Konno T (2001) Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical Q overview of the prototype polymeric drug SMANCS. J. Controlled Release 74:47–61
Schmaljohann D (2006) Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 58:1655–1670
Kim MS, Lee DS (2010) Biodegradable and pH-sensitive polymersome with tuning permeable membrane for drug delivery carrier. Chem Commun 46:4481
Zhou K, Wang Y, Huang X, et al (2011) Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew Chem Int Ed 50:6109–6114
Wu M, Cao Z, Zhao Y, et al (2016) Novel self-assembled pH-responsive biomimetic nanocarriers for drug delivery. Mater Sci Eng C 64:346–353
Lee S-M, Cho J-H, Lee S-D, Kim Y-C (2015) Nanoparticle-encapsulated P2X7 receptor antagonist in a pH-sensitive polymer as a potential local drug delivery system to acidic inflammatory environments. Bioorg Med Chem Lett 25:4197–4202
Torchilin VP (2006) Micellar Nanocarriers: pharmaceutical perspectives. Pharm Res 24:1–16
Ma S-F, Nishikawa M, Katsumi H, et al (2005) Cationic charge-dependent hepatic delivery of amidated serum albumin. J Control Release 102:583–594
Lee HJ, Pardridge WM (2003) Monoclonal antibody radiopharmaceuticals: Cationization, Pegylation, Radiometal chelation, pharmacokinetics, and tumor imaging. Bioconjug Chem 14:546–553
Du J-Z, Sun T-M, Song W-J, et al (2010) A tumor-acidity-activated charge-conversional Nanogel as an intelligent vehicle for promoted Tumoral-cell uptake and drug delivery. Angew Chem 122:3703–3708
Zhou Z, Shen Y, Tang J, et al (2009) Charge-reversal drug conjugate for targeted cancer cell nuclear drug delivery. Adv Funct Mater 19:3580–3589
Gao GH, Lee JW, Nguyen MK, et al (2011) pH-responsive polymeric micelle based on PEG-poly(β-amino ester)/(amido amine) as intelligent vehicle for magnetic resonance imaging in detection of cerebral ischemic area. J Control Release 155:11–17
Weissleder R, Pittet MJ (2008) Imaging in the era of molecular oncology. Nature 452:580–589
Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS (2008) Molecular imaging in drug development. Nat Rev Drug Discov 7:591–607
Weissleder R (2006) Molecular imaging in cancer. Science 312:1168–1171
Feng L, Liu L, Lv F, et al (2014) Preparation and Biofunctionalization of multicolor conjugated polymer nanoparticles for imaging and detection of tumor cells. Adv Mater 26:3926–3930
Chen G, Wang L, Cordie T, et al (2015) Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging. Biomaterials 47:41–50
Song X, Zhang R, Liang C, et al (2015) Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials 57:84–92
Nagireddy NR, Yallapu MM, Kokkarachedu V, et al (2011) Preparation and characterization of magnetic nanoparticles embedded in hydrogels for protein purification and metal extraction. J Polym Res 18:2285–2294
Gao L, Song Q, Huang X, Huang J (2008) A new surfactant-fluorescence probe for detecting shape transitions in self-assembled systems. J Colloid Interface Sci 323:420–425
Acknowledgements
This research was supported by a grant from National Natural Science Foundation of China (NSFC) (Nos. 51473023 and 51103014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, S., Gao, Y., Yu, Z. et al. A pH-triggered charge reversal and self-fluorescent micelle as a smart nanocarrier for doxorubicin controlled release. J Polym Res 24, 94 (2017). https://doi.org/10.1007/s10965-017-1255-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1255-y