Abstract
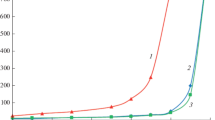
Nonsolvent Induced Phase Separation (NIPS) is among the most well-known methods for membrane fabrication in which the phase separation behavior of the polymer solution is one factor that governs the structure of the membrane ultimately obtained. In this study, phase separation behavior of the polyetherimide (PEI)-casting dope was investigated for different types of coagulants and nonsolvent additives. Cloud point data were obtained by the titration method on the ternary polyetherimide/solvent/coagulant diagram from a limited number of experiments. The whole cloud point curves were then drawn by calculation using the fitting parameters based on the linearized cloud point relation (LCP). In the first part, water, methanol, ethanol, glycerol, and acetic acid were used as the coagulants for the PEI/NMP solution. The cloud point curves obtained for the above coagulants indicated that water has the strongest coagulation power among them. In the second part, methanol, ethanol, glycerol, and acetic acid were used as nonsolvent additives to NMP in different (nonsolvent additive/NMP) mass ratios. The latter (NMP + nonsolvent additive) mixtures were then used as the solvents to prepare PEI/(NMP + nonsolvent additive) solutions. The cloud point data obtained for the above solutions using water as a coagulant indicated that the cloud point curves shift toward the polymer/solution axis as the (nonsolvent additive/NMP) mass ratio increases.

ᅟ









Similar content being viewed by others
Abbreviations
- a:
-
slope of LCP relation
- b:
-
intercept of LCP relation
- v :
-
molar volume of ternary system component (m3 mol−1)
- φ :
-
mass fraction of ternary system component
- δ d :
-
dispersive component of the solubility parameter (J/cm3)0.5
- δ p :
-
polar component of the solubility parameter (J/cm3)0.5
- δ h :
-
hydrogen-bonding contribution to the solubility parameter (J/cm3)0.5
- Δδ i − p :
-
solubility parameter difference
References
Peng N, Chung TS, Wang KY (2008) Macrovoid evolution and critical factors to form macrovoid-free hollow fiber membranes. Journal of Membrane Science 318:363–372
Kesting RE, Fritzsche AK, Cruse A, Murphy MK, Handermann AC, Malon RF, Moore MD (1990) The second generation polysulfone gas-separation membranes. I: the use of Lewis acid:base complexes as transient templates to increase free volume. J Appl Polym Sci 40:1557–1574
Ren JZ, Li ZS, Wong FS (2004) Membrane structure control of BTDA-TDI/MDI (P84) co-polyimide asymmetric membranes by wet-phase inversion process. J Membr Sci 241:305–314
Li DF, Chung TS, Wang R (2004) Morphological aspects and structure control of dual-layer asymmetric hollow fiber membranes formed by a simultaneous coextrusion approach. J Membr Sci 243:155–175
Kim JH, Min BR, Won J, Park HC, Kang YS (2001) Phase behavior and mechanism of membrane formation for polyimide/DMSO/water system. J Membr Sci 187:47–55
Ren JZ, Chung TS, Li DF, Wang R, Liu Y (2002) Development of asymmetric 6FDA-2,6 DAT hollow fiber membranes for CO2/CH4 separation: 1. The influence of dope composition and rheology on membrane morphology and separation performance. J Membr Sci 207:227–240
Altena FW, Smolders CA (1982) Calculation of liquid-liquid phase separation in a ternary system of a polymer in a mixture of a solvent and a nonsolvent. Macromolecules 15(6):1491–1497
Wang D, Li K, Teo WK (2000) Porous PVDF asymmetric hollow fiber membranes prepared with the use of small molecular additives. J Membr Sci 178:13–23
Mansourizadeh A, Ismail AF (2012) Influence of membrane morphology on characteristics of porous hydrophobic PVDF hollow fiber contactors for CO2 stripping from water. Desalination 287:220–227
Wei YM, Xu ZL, Yang XT, Liu HL (2006) Mathematical calculation of binodal curves of a polymer/solvent/nonsolvent system in the phase inversion process. Desalination 192:91–104
Yilmaz L, McHugh AJ (1986) Analysis of nonsolvent-solvent-polymer phase diagrams and their relevance to membrane formation modeling. J Appl Polym Sci 31(4):997–1018
Sanchez IC, Panayiotou CG (1994) Equations of state thermodynamics of polymer and related solutions. Marcel & Dekker, New York
Economou IG (2002) Statistical associating fluid theory: a successful model for the calculation of thermodynamic and phase equilibrium properties of complex fluid mixtures. Ind Eng Cham Res 41(5):953–962
Gonzalez-Leon JA, Mayes AM (2003) Phase behavior prediction of ternary polymer mixtures. Macromolecules 36:2508–2515
Maghsoud Z, Navid Famili MH, Madaeni SS (2010) Phase diagram calculations of water/tetrahydrofuran/poly(vinyl chloride) ternary system based on a compressible regular solution model, Iran. Polym J 19(8):581–588
Boom RM, van den Boomgaard T, van den Berg JWA, Smolders CA (1993) Linearized cloudpoint curve correlation for ternary systems consisting of one polymer, one solvent and one non-solvent. Polymer 34:2348–2356
Shen LQ, Xu ZK, Xu YY (2003) Phase separation behavior of poly(ether imide)/N, N-dimethylacetamide/nonsolvent systems. J Appl Polym Sci 89:875–881
Lau WWY, Cuiver MD, Matsuura T (1991) Phase separation in carboxylated polysulfone/solvent/water systems. J Appl Polym Sci 42(12):3215–3221
Ismail AF, Matsuura T (2012) Sustainable membrane technology for energy, water, and environment. John Wiley & Sons Inc., Hoboken, New Jersey
Wang D, Li K, Teo WK (1999) Phase separation in polyetherimide/solvent/nonsolvent systems and membrane formation. J Appl Polym Sci 71:1789–1796
Tasselli F, Drioli E (2007) Tuning of hollow fiber membrane properties using different bore fluids. J Membr Sci 301:11–18
Shi L, Wang R, Cao Y, Feng C, Liang DT, Tay JH (2007) Fabrication of poly(vinylidene fluoride-co-hexafluropropylene) (PVDF-HFP) asymmetric microporous hollow fiber membranes. J Membr Sci 305:215–225
Wienk IM, Boom RM, Beerlage MAM, Bulte AMW, Smolders CA, Strathmann H (1996) Recent advances in the formation of phase inversion membranes made from amorphous or semi-crystalline polymers. J Membr Sci 113:361–371
Macchionea M, Jansena JC, Drioli E (2006) The dry phase inversion technique as a tool to produce highly efficient asymmetric gas separation membranes of modified PEEK. Influence of temperature and air circulation. Desalination 192:132–141
Ren J, Zhou J, Deng M (2010) Morphology transition of asymmetric flat sheet and thickness-gradient membranes by wet phase-inversion process. Desalination 253:1–8
Cranford RJ, Darmstadt H, Yang J, Roy C (1999) Polyetherimide/polyvinylpyrrolidone vapor permeation membranes. Physical and chemical characterization. J Membr Sci 155:231–240
Wang D, Teo WK, Li K (2002) Permeation of H2, N2, CH4, C2H6, and C3H8 through asymmetric polyetherimide hollow-fiber membranes. J Appl Polym Sci 86:698–702
Wang D, Li K, Teo WK (1998) Preparation and characterization of polyetherimide asymmetric hollow fiber membranes for gas separation. J Membr Sci 138:193–201
Kurdi J, Tremblay AY (2001) The influence of casting solution structure on the microporosity of polyetherimide gas separation membranes prepared by the coagulation post-leaching method. J Membr Sci 184:175–186
Wang D, Li K, Teo WK (2002) Short communication, Preparation of asymmetric polyetherimide hollow fiber membrane with high gas selectivities. J Membr Sci 208:419–426
Bakeri G, Matsuura T, Ismail AF (2011) The effect of phase inversion promoters on the structure and performance of polyetherimide hollow fiber membrane using in gas–liquid contacting process. J Membr Sci 383:159–169
Xu ZK, Shen LQ, Yang Q, Liu F, Wang SY, Xu YY (2003) Ultrafiltration hollow fiber membranes from poly(ether imide): preparation, morphologies and properties. J Membr Sci 223:105–118
Kim IC, Yoon HG, Lee KH (2002) Formation of integrally skinned asymmetric polyetherimide nanofiltration membranes by phase inversion process. J Appl Polym Sci 84:1300–1307
Feng X, Huang RYM (1996) Preparation and performance of asymmetric polyetherimide membranes for isopropanol dehydration by pervaporation. J Membr Sci 109:165–172
Xu ZL, Chung TS, Huang Y (1999) Effect of polyvinylpyrrolidone molecular weights on morphology, oil/water separation, mechanical and thermal properties of polyetherimide/polyvinylpyrrolidone hollow fiber membranes. J Appl Polym Sci 74:2220–2233
Law SJ, Mukhopadhyay SK (1997) The construction of a phase diagram for a ternary system used for the wet spinning of acrylic fibers based on a linearized cloudpoint curve correlation. J Appl Polym Sci 65:2131–2139
Zhang J, Zhang Y, Zhao J (2011) Thermodynamic study of non-solvent/dimethyl sulfoxide/polyacrylonitrile ternary systems: effects of the non-solvent species. Polym Bull 67:1073–1089
Dong R, Zhao J, Zhang Y, Pan D (2009) Morphology control of polyacrylonitrile (PAN) fibers by phase separation technique. J Polym Sci, Part B: Polym Phys 47:261–275
Wang D, Li K, Sourirajan S, Teo WK (1993) Phase separation phenomena of polysulfone/solvent/organic nonsolvent and polyethersulfone/solvent/organic nonsolvent systems. J Appl Polym Sci 50:1693–1700
Ren J, Zhou J, Deng M (2010) Morphology transition of asymmetric polyetherimide flat sheet membranes with different thickness by wet phase-inversion process. Sep Purif Technol 74:119–129
Liu B, Du Q, Yang Y (2000) The phase diagrams of mixtures of EVAL and PEG in relation to membrane formation. J Membr Sci 180:81–92
Guillen GR, Pan Y, Li M, Hoek EMV (2011) Preparation and characterization of membranes formed by nonsolvent induced phase separation: a review. Ind Eng Chem Res 50:3798–3817
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bakeri, G., Ismail, A.F., Rahimnejad, M. et al. Analysis of Polyetherimide/N-Methyl-2-Pyrrolidone/nonsolvent phase separation behavior. J Polym Res 21, 386 (2014). https://doi.org/10.1007/s10965-014-0386-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0386-7