Abstract
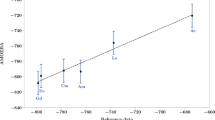
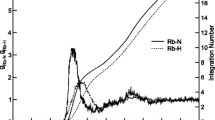
Using fragment molecular orbital–molecular dynamics (FMO–MD) simulation at the FMO3-HF/6-31G(d,p) level, the hydration of a Ra2+ ion was theoretically investigated. The first peaks of the radial distribution function (RDF) for Ra–O and Ra–H lengths were predicted to be 2.85 and 3.45 Å with broad envelopes in the ranges of 2.5–3.5 and 2.8–4.3 Å, respectively. The broad peaks shows that the first hydration shell of Ra2+ is much more flexible than those in the other hydrated divalent alkaline earth metal ions, i.e., Ra2+ is a structure-breaking ion. The hydration number of Ra2+ was predicted to be 8.1. From the angular distribution function (ADF), it was clarified that the octa hydrated Ra2+ ion has a flexible square antiprism structure at room temperature.



Similar content being viewed by others
References
Ohtaki, H., Radnai, T.: Structure and dynamics of hydrated ions. Chem. Rev. 93, 1157–1204 (1993)
Park, J., Kolaski, M., Lee, H.M., Kim, K.S.: Insights into the structures, energetics, and vibrations of aqua-rubidium(I) complexes: ab initio study. J. Chem. Phys. 121, 3108–3116 (2004)
Hofer, T.S., Randolf, B.R., Rode, B.M.: Structure-breaking effects of solvated Rb(I) in dilute aqueous solution: an ab initio QM/MM MD approach. J. Comput. Chem. 26, 949–956 (2005)
Kolaski, M., Lee, H.M., Choi, Y.C., Kim, K.S., Tarakeshwar, P., Miller, D.J., Lisy, J.M.: Structures, energetics, and spectra of aqua-cesium(I) complexes: an ab initio and experimental study. J. Chem. Phys. 126, 074302 (2007)
Schwenk, C.F., Hofer, T.S., Rode, B.M.: Structure breaking effect of hydrated Cs+. J. Phys. Chem. A 108, 1509–1514 (2004)
Spohr, E., Palinkas, G., Heinzinger, K., Bopp, P., Probst, M.M.: Molecular dynamics study of an aqueous strontium chloride solution. J. Phys. Chem. 92, 6754–6761 (1988)
Hofer, T.S., Randolf, B.R., Rode, B.M.: Sr(II) in water: a labile hydrate with a highly mobile structure. J. Phys. Chem. B 110, 20409–20417 (2006)
Pfund, D.M., Darab, J.G., Fulton, J.L., Ma, Y.: An XAFS study of strontium ions and krypton in supercritical water. J. Phys. Chem. 98, 13102–13107 (1994)
Ramos, S., Neilson, G.W., Barnes, A.C., Capitn, M.J.: Anomalous X-ray diffraction studies of Sr2+ hydration in aqueous solution. J. Chem. Phys. 118, 5542–5546 (2003)
Hofer, T.S., Rode, B.M., Randolf, B.R.: Structure and dynamics of solvated Ba(II) in dilute aqueous solution: an ab initio QM/MM–MD approach. Chem. Phys. 312, 81–88 (2005)
D’Angelo, P., Pavel, N.V., Roccatano, D., Nolting, H.F.: Multielectron excitations at the l edges of barium in aqueous solution. Phys. Rev. B 54, 12129–12138 (1996)
Fujiwara, T., Mochizuki, Y., Komeiji, Y., Okiyama, Y., Mori, H., Nakano, T., Miyoshi, E.: Fragment molecular orbital-based molecular dynamics (FMO–MD) simulations on hydrated Zn(II) ion. Chem. Phys. Lett. 490, 41–45 (2010)
Fujiwara, T., Mori, H., Mochizuki, Y., Tatewaki, H., Miyoshi, E.: Theoretical study of hydration models of trivalent rare-earth ions using model core potentials. J. Mol. Struct. 949, 28–35 (2010)
Fujiwara, T., Mori, H., Mochizuki, Y., Osanai, Y., Miyoshi, E.: 4f-in-core model core potentials for trivalent lanthanides. Chem. Phys. Lett. 510, 261–266 (2011)
Ayala, R., Martinez, J.M., Pappalardo, R.R., Munoz-Paez, A., Marcos, E.S.: Po(IV) hydration: a quantum chemical study. J. Phys. Chem. B 112, 5416–5422 (2008)
Lutz, O.M.D., Hofer, T.S., Randolf, B.R., Weiss, A.K.H., Rode, B.M.: A qmcf–md investigation of the structure and dynamics of Ce4+ in aqueous solution. Inorg. Chem. 51, 6746–6752 (2012)
Glendening, E.D., Feller, D.: Dicationwater interactions: m2+(H2O) n clusters for alkaline earth metals M = Mg, Ca, Sr, Ba, and Ra. J. Phys. Chem. 100, 4790–4797 (1996)
Han, Y.K., Jeong, H.Y.: Comment on dication water interactions: M2+(H2O) n clusters for alkaline earth metals M = Mg, Ca, Sr, Ba, and Ra. J. Phys. Chem. 100, 18004–18005 (1996)
Matsuda, A., Mori, H.: A quantum chemical study on hydration of Ra(II): comparison with the other hydrated divalent alkaline earth metal ions. J. Comp. Chem. Jpn. 14, 1–13 (2014)
Caminiti, R., Licheri, G., Piccaluga, G., Pinna, G.: X-ray diffraction study of a three-ion aqueous solution. Chem. Phys. Lett. 47, 275–278 (1977)
Hewish, N.A., Enderby, J.E., Howells, W.S.: The dynamics of water molecules in ionic solution. J. Phys. C 16, 1777–1791 (1983)
Matwiyoff, N.A., Taube, H.: Direct determination of the solvation number of magnesium(II) ion in water, aqueous acetone, and methanolic acetone solutions. J. Am. Chem. Soc. 90, 2796–2800 (1968)
Tongraar, A., Rode, B.M.: The role of non-additive contributions on the hydration shell structure of Mg2+ studied by Born–Oppenheimer ab initio quantum mechanical/molecular mechanical molecular dynamics simulation. Chem. Phys. Lett. 346, 485–491 (2001)
Tongraar, A., Liedl, K.R., Rode, B.M.: Solvation of Ca2+ in water studied by Born–Oppenheimer ab initio QM/MM dynamics. J. Phys. Chem. A 101, 6299–6309 (1997)
Bernal-Uruchurtu, M.I., Ortega-Blake, I.: A refined Monte Carlo study of Mg2+ and Ca2+ hydration. J. Chem. Phys. 103, 1588–1598 (1995)
Katz, A.K., Glusker, J.P., Beebe, S.A., Bock, C.W.: Calcium ion coordination: a comparison with that of beryllium, magnesium, and zinc. J. Am. Chem. Soc. 118, 5752–5763 (1996)
Komeiji, Y., Uebayasi, M., Takata, R., Shimizu, A., Itsukashi, K., Taiji, M.: Fast and accurate molecular dynamics simulation of a protein using a special-purpose computer. J. Comput. Chem. 18, 1546–1563 (1997)
Nakano, T., Kaminuma, T., Sato, T., Fukuzawa, K., Akiyama, Y., Uebayasi, M., Kitaura, K.: Fragment molecular orbital method: use of approximate electrostatic potential. Chem. Phys. Lett. 351, 475–480 (2002)
Dill, J.D., Pople, J.A.: Selfconsistent molecular orbital methods. XV. Extended Gaussian-type basis sets for lithium, beryllium, and boron. J. Chem. Phys. 62, 2921–2923 (1975)
Hehre, W.J., Ditchfield, R., Pople, J.A.: Selfconsistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972)
Anjima, H., Tsukamoto, S., Mori, H., Mine, M., Klobukowski, M., Miyoshi, E.: Revised model core potentials of s-block elements. J. Comput. Chem. 28, 2424–2430 (2007)
Miyoshi, E., Mori, H., Hirayama, R., Osanai, Y., Noro, T., Honda, H., Klobukowski, M.: Compact and efficient basis sets of s- and p-block elements for model core potential method. J. Chem. Phys. 122, 074104 (2005)
Osanai, Y., Mon, M.S., Noro, T., Mori, H., Nakashima, H., Klobukowski, M., Miyoshi, E.: Revised model core potentials for first-row transition-metal atoms from Sc to Zn. Chem. Phys. Lett. 452, 210–214 (2008)
Osanai, Y., Soejima, E., Noro, T., Mori, H., Mon, M.S., Klobukowski, M., Miyoshi, E.: Revised model core potentials for second-row transition metal atoms from Y to Cd. Chem. Phys. Lett. 463, 230–234 (2008)
Mori, H., Ueno-Noto, K., Osanai, Y., Noro, T., Fujiwara, T., Klobukowski, M., Miyoshi, E.: Revised model core potentials for third-row transitionmetal atoms from Lu to Hg. Chem. Phys. Lett. 476, 317–322 (2009)
Mori, H., Zeng, T., Klobukowski, M.: Assessment of chemical core potentials for the computation on enthalpies of formation of transition-metal complexes. Chem. Phys. Lett. 521, 150–156 (2012)
Halgren, T.A.: Merck molecular force field. V. Extension of MMFF94 using experimental data, additional computational data, and empirical rules. J. Comput. Chem. 17, 616–641 (1996)
MOE (the molecular operating environment) version 2009.10; software available from Chemical Computing Group, Inc.: 1010 Sherbrooke Street West, suite 910, Montreal, Canada H3A 2R7; http://www.chemcomp.com
Komeiji Y., Mochizuki Y., Nakano T., Mori H., Chap. X: Recent advances in fragment molecular orbital-based molecular dynamics (FMO–MD) simulation in molecular dynamics. In: Wang, L. (ed.) Molecular dynamics—theoretical developments and Applications in Nanotechnology and Energy. Intech (2012); ISBN 979-953-307-615-6, www.intechopen.com
Mori, H., Hirayama, N., Komeiji, Y., Mochizuki, Y.: Differences in hydration between cis- and trans-platin: quantum insights by ab initio fragment molecular orbital-based molecular dynamics (FMO–MD). Comp. Theor. Chem. 986, 30–34 (2012)
Martyna, G.J., Klein, M.L., Tuckerman, M.: Nosé–Hoover chains: the canonical ensemble via continuous dynamics. J. Chem. Phys. 97, 2635–2643 (1992)
Hedström, H., Persson, I., Skarnemark, G., Ekberg, C.: Characterization of radium sulphate. J. Nucl. Chem., article number 940701 (2013)
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number 25810002 and Yamada Science Foundation. AM is also grateful to JSPS for the Research Fellowships for Young Scientists. A part of the calculations reported here were performed using computing resources in the Research Center for Computational Science, Okazaki, Japan. The authors would thank Dr. Yuto Komeiji, and Prof. Yuji Mochizuki for fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuda, A., Mori, H. Theoretical Study on the Hydration Structure of Divalent Radium Ion Using Fragment Molecular Orbital–Molecular Dynamics (FMO–MD) Simulation. J Solution Chem 43, 1669–1675 (2014). https://doi.org/10.1007/s10953-014-0235-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0235-7