Abstract
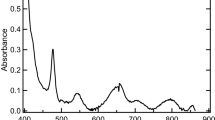
Chromium(III)-phosphate reactions are expected to be important in managing high-level radioactive wastes stored in tanks at many DOE sites. Extensive studies on the solubility of amorphous Cr(III) solids in a wide range of pH (2.8–14) and phosphate concentrations (10−4 to 1.0 m) at room temperature (22±2)°C were carried out to obtain reliable thermodynamic data for important Cr(III)-phosphate reactions. A combination of techniques (XRD, XANES, EXAFS, Raman spectroscopy, total chemical composition, and thermodynamic analyses of solubility data) was used to characterize solid and aqueous species. Contrary to the data recently reported in the literature,(1) only a limited number of aqueous species [Cr(OH)3H2PO−4, Cr(OH)3(H2PO4)2−2), and Cr(OH)3HPO2−4] with up to about four orders of magnitude lower values for the formation constants of these species are required to explain Cr(III)-phosphate reactions in a wide range of pH and phosphate concentrations. The log Ko values of reactions involving these species [Cr(OH)3(aq)+H2PO−4⇌Cr(OH)3H2PO−4; Cr(OH)3(aq)+2H2PO−4⇌Cr(OH)3(H2PO4)2−2; Cr(OH)3(aq)+HPO2−4⇌Cr(OH)3HPO2−4] were found to be 2.78±0.3, 3.48±0.3, and 1.97±0.3, respectively.
Similar content being viewed by others
References
S. E. Ziemniak, M. E. Jones, and K. E. S. Combs, J. Solution Chem. 27,33–66 (1998).
P. Hrma, J. Vienna, J. Crum, G. Piepel, and M. Mika, Mat. Res. Soc. Proc. 608,671–676 (2000).
J. L. Swanson, Clean Option: An Alternative Strategy for Hanford Tank Waste Remediation. Vol. 2: Detailed Description of First Example Flowsheet. PNL-8288 (Pacific Northwest National Laboratory, Richland, WA, 1993).
M. J. Kupfer, A. L. Boldt, K. M. Hodgson, L. W. Shelton, B. C. Simpson, R. A. Watrous, B. A. Higley, R. M. Orme, M. D. LeClair, G. L. Borsheim, R. T. Winward, N. G. Colton, S. L. Lambert, D. E. Place, and W. W. Schultz, Standard Inventories of Chemicals and Radionuclides in Hanford Site Tanks. HNF-SD-WM-TI-740 (Lockheed Martin Hanford Company, Richland, WA, 1998).
J. D. Anderson, A History of the 200 Area Tank Farms. WHC-MR0132 (Westinghouse Hanford Company, Richland, WA, 1990).
D. Rai, N. J. Hess, L. Rao, Z. Zhang, A. R. Felmy, D. A. Moore, S. B. Clark, and G. Lumetta, J. Solution Chem. 31,343–367 (2002).
F. G. Zharovskii, Trudy Kowissii Analit. Khim. Akad. Nau k. SSSR 3, 101 (1951). (quoted by L. G. Sillen and A. E. Martell, Stability Constants of Metal-Ion Complexes. Special Publication No. 17. The Chemical Society, London)
S. C. Lahiri and S. Aditya, J. Indian Chem. Soc. 43,513–517 (1966).
A. E. Aleshechkina, V. M. Masalovich, P. K. Agasyan, and B. P. Sereda, Russ. J. Inorg. Chem. 21,973–975 (1976).
D. Rai, B. M. Sass, and D. A. Moore, Inorg. Chem. 26,345–349 (1987).
S. M. Sterner, A. R. Felmy, J. R. Rustad, and K. S. Pitzer, Thermodynamic Analysis of Aqueous Solutions Using INSIGHT. PNWD-SA-4436 (Pacific Northwest National Laboratory, Richland, WA, 1997).
R. C. Weast, Handbook of Chemistry and Physics, 53rd edn. (The Chemical Rubber Co., Cleveland, OH, 1972).
D. Rai, N. J. Hess, A. R. Felmy, D. A. Moore, M. Yui, and P. Vitorge, Radiochim. Acta 86,89–99 (1999).
K. S. Pitzer and G. Mayorga, J. Phys. Chem. 77,2300–2308 (1973).
K. S. Pitzer, Ion Interaction Approach: Theory and Data Correlation, Chap. 3 (CRC Press, Boca Ratan, FL, 1991).
A. R. Felmy, D. Rai, J. A. Schramke, and J. L. Ryan, Radiochim. Acta 48,29–35 (1989).
D. Rai, A. R. Felmy, and R. W. Szelmeczka, J. Solution Chem. 20,375–390 (1991).
D. Rai, A. R. Felmy, S. M. Sterner, D. A. Moore, M. J. Mason, and C. F. Novak, Radiochim. Acta 79,239–247 (1997).
D. Rai, Y. Xia, N. J. Hess, D. M. Strachan, and B. P. McGrail, J. Solution Chem. 30,949–967 (2001).
I. R. Beattie and T. R. Gilson, J. Chem. Soc. A. 980,(1970).
G. M. Begun, G. W. Beall, L. A. Boatner, and W. J. Gregor, J. Raman Spectrosc. 11,273 (1981).
K. S. Pitzer and L. F. Silvester, J. Solution Chem. 5,(1976).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, D., Moore, D.A., Hess, N.J. et al. Chromium(III) Hydroxide Solubility in The Aqueous Na+-OH−-H2PO−4-HPO2−4-PO3−4-H2O System: A Thermodynamic Model. J Solution Chem 33, 1213–1242 (2004). https://doi.org/10.1007/s10953-004-7137-z
Issue Date:
DOI: https://doi.org/10.1007/s10953-004-7137-z