Abstract
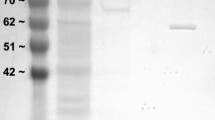
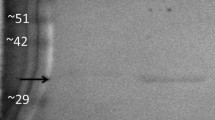
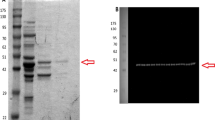
Lactate dehydrogenase (LDH) has a crucial role in maintaining ATP production as the terminal enzyme in anaerobic glycolysis. This study will determine the effect of posttranslational modifications (PTMs) on the activity of LDH in the foot muscle and hepatopancreas of an estivating snail, Otala lactea. LDH in foot muscle of O. lactea was purified to homogeneity and partially purified in hepatopancreas in a two-step and three-step process, respectively. The kinetic properties and stability of these isoforms were determined where there was a significant difference in Km and I50 values with pyruvate and urea separately in foot muscle; however, hepatopancreas exhibited significant differences in Km and I50 in salt between control and stress. Interestingly, hepatopancreas has a higher affinity for pyruvate in the control state whereas foot muscle has a higher affinity for its substrate in the estivated state. PTMs of each isoform were identified using immunoblotting and dot blots, which prove to be significantly higher in the control state. Overall, foot muscle LDH enters a low phosphorylation state during estivation allowing more efficiency in consuming pyruvate with higher thermal stability but less structural stability. Hepatopancreas LDH becomes dephosphorylated in the estivating snail that decreases the efficiency of the enzyme in the forward direction; however, the snail has an increased tolerance to the presence of salt when water becomes scarce. Such tissue-specific regulations indicate the organism’s ability to reduce energy consumption when undergoing metabolic depression.






Similar content being viewed by others
Abbreviations
- LDH:
-
Lactate dehydrogenase
- PTMs:
-
Posttranslational modifications
- PMSF:
-
Phenylmethylsulfonyl fluoride
- NAD:
-
Nicotinamide adenine dinucleotide
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TBST:
-
Tris-buffered saline and tween
- TBS:
-
Tris-buffered saline
References
Ramnanan CJ, McMullen DC, Groom AG, Storey KB (2010) The regulation of AMPK signaling in a natural state of profound metabolic rate depression. Mol Cell Biochem 335:91–105
Whitwam RE, Storey KB (1990) Pyruvate kinase from the land snail Otala lactea: regulation by reversible phosphorylation during estivation and anoxia. J Exp Biol 154:321–337
Bishop T, Brand MD (2000) Processes contributing to metabolic depression in hepatopancreas cells from the snail Helix aspersa. J Exp Biol 20:3603–3612
Brooks SPJ, Storey KB (1997) Glycolytic controls in estivation and anoxia: a comparision of metabolic arrest in land and marine molluscs. Comp Biochem Physiol A 118:1103–1114
Ramos-Vasconcelos GR, Hermes-Lima M (2003) Hypometabolism, antioxidant defenses and free radical metabolism in the pulmonate land snail Helix aspersa. J Exp Biol 206:675–685
Ramnanan CJ, Allan ME, Groom AG, Storey KB (2009) Regulation of global protein translation and protein degradation in aerobic dormancy. Mol Cell Biochem 323:9–20
Herreid CF (1977) Metabolism of land snails (Otala lactea) during dormancy, arousal and activity. Comp Biochem Physiol A 56:211–215
Whitwam RE, Storey KB (1991) Regulation of phosphofructokinase during estivation and anoxia in the land snail, Otala lactea. Physiol Zool 64:595–610
Livingstone DR, de Zwaan A (1983) In: Wilbur KM (ed) Carbohydrate metabolism in gastropods. Academic Press, New York
Umezurike GM, Iheanacho EN (1983) Metabolic adaptations in aestivating giant African snail (Achatina achatina). Comp Biochem Physiol B 74:493–498
Barnhart C (1986) Control of acid-base status in active and dormant land snails, Otala lactea (Pulmonata, Helicidae). J Comp Physiol B 156:347–354
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York
Storey KB (1988) Suspended animation: the molecular basis of metabolic depression. Can J Zool 66:124–132
Abboud J, Storey KB (2013) Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. PeerJ 1:e12. doi:10.7717/peerj.12
Xiong ZJ, Storey KB (2012) Regulation of liver lactate dehydrogenase by reversible phosphorylation in response to anoxia in a freshwater turtle. Comp Biochem Physiol B 163:221–228
Dawson NJ, Katzenback BA, Storey KB (2014) Free-radical first responders: the characterization of CuZnSOD and MnSOD regulation during freezing of the freeze-tolerant North American wood frog, Rana sylcatica. Biochim Biophys Acta 1850:97–106
Cohen P (2002) The origins of protein phosphorylation. Nat Cell Biol 4:E127–E130
Brooks SPJ (1992) A simple computer program for the analysis of enzyme kinetics. Biotechniques 13:906–911
Celis JE, Carter N, Hunter T, Simons K, Small JV, Shotton D (2006) Cell biology a laboratory handbook, 3rd edn. Elsevier Science, New York
Dawson NJ, Bell RA, Store KB (2013) Purification and properties of white muscle lactate dehydrogenase from the anoxia-tolerant turtle, the red-eared slider, Trachemys scripta elegans. Enzyme Res. doi:10.1155/2013/784973
Biggar KK, Dawson NJ, Storey KB (2012) Real-time protein unfolding: a method for determining the kinetics of native protein denaturation using a quantitative real-time thermocycler. Biotechniques 5:231–238
Storey KB, Storey JM (2007) Putting life on “pause”—molecular regulation of hypometabolism. J Exp Biol 210:1700–1714
Brooks SPJ, Storey KB (1995) Protein phosphorylation patterns during aestivation in the land snail Otala lactea. Mol Cell Biochem 143:7–13
Ramnanan CJ, Storey KB (2006) Glucose-6-phosphate dehydrogenase regulation during hypometabolism. Biochem Biophys Res Commun 339:7–16
Childers CL, Storey KB (2016) Post-translational regulation of hexokinase function and protein stability in the aestivating frog Xenopus laevis. Protein J 35:61–71
Ramnanan CJ, Storey KB (2009) Regulation of type-1 protein phosphatase in a model of metabolic arrest. Comp Biochem Physiol B 148:245–255
Yasykova MY, Petukhov SP, Muronetz VI (2000) Phosphorylation of lactate dehydrogenase by protein kinases. Biochem Mosc 65:1192–1196
Bedford MT, Richard S (2005) Arginine methylation an emerging regulator of protein function. Mol Cell 18:263–272
Botting CH, Talbot P, Paytubi S, White MF (2010) Extensive lysine methylation in hyperthermophilic crenarchaea: potential implications for protein stability and recombinant enzymes. Archaea. doi:10.1155/2010/106341
Lanouette S, Mongeon V, Figeys D, Couture JF (2014) The functional diversity of protein lysine methylation. Mol Syst Biol 10:724
Ramnanan CJ, Storey KB (2006) Suppression of Na+/K+-ATPase activity during estivation in the land snail Otala lactea. J Exp Biol 209:677–688
Ramnanan CJ, Storey KB (2007) The regulation of thapsigargin-sensitive sarcoendoplasmic reticulum Ca(2+)-ATPase activity in estivation. J Comp Physiol 178:33–45
Ramnanan CJ, Groom AG, Storey KB (2007) Akt and its downstream targets play roles in mediating dormancy in land snails. BMB Rep 42:817–822
Childers CL, Green SR, Dawson NJ, Storey KB (2016) Native denaturation differential scanning fluorimetry: determining the effect of urea using a quantitative real-time thermocycler. Anal Biochem 508:114–117
Read JA, Winter VJ, Eszes CM, Sessions RB, Brady RL (2001) Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins 43:175–185
Riddle WA (1975) Water relations and humidity-related metabolism of the desert snail Rabdotus schiedeanus (Pfeiffer) (Helicidae). Comp Biochem Physiol A 5:579–583
Schimdt-Nielsen K, Taylor CR, Shkolnik A (1971) Desert snails: problems of heat, water and food. J Exp Biol 55:385–398
Schimdt-Nielsen K, Taylor CR, Shkolnik A (1972) Desert snails: problems of survival. Symp Zool Soc Lond 31:1–13
Yom-Tov Y (1971) Body temperature and light reflectance in two desert snails. Proc Malacol Soc Lond 39:319–326
Campbell JW, Drotman RB, McDonall JA, Tramell PR (1972) In: Campbell JW, Goldstein L (eds) Nitrogen metabolism in terrestrial invertebrates. Academic Press, New York
Brooks SPJ, Storey KB (1992) Properties of pyruvate dehydrogenase from the land snail, Otala lactea: control of enzyme activity during estivation. Physiol Zool 65:620–633
Grundy JE, Storey KB (1994) Urea and salt effects on enzymes from estivating and non estivating amphibians. Mol Cell Biochem 131:9–17
Lin H, Yang Y, Quan R, Mendoza I, Wu Y, Du W, Zhao S, Schumaker KS, Pardo JM, Guo Y (2009) Phosphorylation of SOS3-like calcium binding proteins by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21:1607–1619
Acknowledgements
The authors wish to thank C.L. Childers for editorial review of the manuscript. This research was supported by a discovery grant (6793) from the Natural Sciences and Engineering Research Council (NSERC) of Canada to KBS and the Canada Research Chairs program. A.M.S. Mattice and I.A. MacLean both hold NSERC Undergraduate Student Research Awards.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
This article does not contain any studies with human participants or vertebrate animals performed by any of the authors.
Additional information
Isabelle A. MacLean and Amanda M. S. Mattice are the primary authors and have contributed equally.
Rights and permissions
About this article
Cite this article
MacLean, I.A., Mattice, A.M.S., Adam, N.J. et al. Purification and Characterization of Lactate Dehydrogenase in the Foot Muscle and Hepatopancreas of Otala lactea . Protein J 35, 467–480 (2016). https://doi.org/10.1007/s10930-016-9689-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-016-9689-3