Abstract
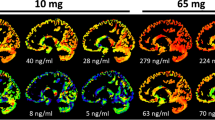
An understanding of the relationship between drug exposure and response is a fundamental basis for any dosing recommendation. We investigate optimal dose-selection for two different types of studies, a receptor occupancy study assessed by positron emission tomography (PET) and a dose-finding study in neuropathic pain treatment. For the PET-study, an inhibitory E-max model describes the relationship between drug exposure and displacement of a radioligand from specific receptors in the brain. The model has a mechanistic basis in the law of mass action and the affinity parameter (Ki PL ) is of primary interest. For optimization of the neuropathic pain study, the model is empirical and the exposure response curve itself is of primary interest. An alternative parameterization of the sigmoid Emax model was therefore used where the plasma concentration corresponding to the minimum relevant efficacy was estimated as a parameter. Optimal design methodology was applied using the D-optimal criterion as well as the Ds-optimal criterion where parameters of interest were defined. For the PET-study it was shown that the precision of Ki PL can be improved by inclusion of brain regions with both high and low receptor density and that the need for high doses is reduced when a brain region with low receptor density is included in the analysis. In the case of the neuropathic pain study it was shown that a Ds-optimal study design using the reparameterized Emax model can improve the precision in the minimum effective dose compared to a D-optimal design.






Similar content being viewed by others
References
International Conference of Harmonisation (ICH) (1994) Dose-response information to support drug registration E4
Food and Drug Administration (FDA) (2003) Guidance for industry: exposure-response relationships—study design, data analysis and regulatory applications
Pinheiro J, Bornkamp B, Glimm E, Bretz F (2013) Model-based dose finding under model uncertainty using general parametric models. Stat Med. doi:10.1002/sim.6052
Miller F, Guilbaud O, Dette H (2007) Optimal designs for estimating the interesting part of a dose-effect curve. J Biopharm Stat 17(6):1097–1115. doi:10.1080/10543400701645140
Bretz F, Hsu J, Pinheiro J, Liu Y (2008) Dose finding—a challenge in statistics. Biometr J 50(4):480–504. doi:10.1002/bimj.200810438
Dette H, Bretz F, Pepelyshev A, Pinheiro J (2008) Optimal designs for dose-finding studies. J Am Stat Assoc 103(483):1225–1237. doi:10.1198/016214508000000427
Kagedal M, Cselenyi Z, Nyberg S, Raboisson P, Stahle L, Stenkrona P, Varnas K, Halldin C, Hooker AC, Karlsson MO (2013) A positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066—estimating occupancy in the absence of a reference region. NeuroImage 82:160–169. doi:10.1016/j.neuroimage.2013.05.006
Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, Osman S, Bloomfield PM, Jones T, Patsalos PN et al (1995) Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab 15(1):152–165. doi:10.1038/jcbfm.1995.17
Farde L, Hall H, Ehrin E, Sedvall G (1986) Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 231(4735):258–261
Lockwood PA, Cook JA, Ewy WE, Mandema JW (2003) The use of clinical trial simulation to support dose selection: application to development of a new treatment for chronic neuropathic pain. Pharm Res 20(11):1752–1759
Holford NH, Sheiner LB (1981) Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet 6(6):429–453
Groth AV (2008) Alternative parameterisations of saturable (Emax) models allowing for nesting of non-saturable models. Population Approach Group Europe (PAGE) 17:Abstr 1371
Nyberg J, Ueckert S, Stromberg EA, Hennig S, Karlsson MO, Hooker AC (2012) PopED: an extended, parallelized, nonlinear mixed effects models optimal design tool. Comput Methods Progr Biomed 108(2):789–805. doi:10.1016/j.cmpb.2012.05.005
Foracchia M, Hooker A, Vicini P, Ruggeri A (2004) POPED, a software for optimal experiment design in population kinetics. Comput Methods Progr Biomed 74(1):29–46. doi:10.1016/S0169-2607(03)00073-7
Ueckert S, Hennig S, Nyberg J, Karlsson MO, Hooker AC (2013) Optimizing disease progression study designs for drug effect discrimination. J Pharmacokinet Pharmacodyn 40(5):587–596. doi:10.1007/s10928-013-9331-3
Atkinson A, Donev AN (1992) Optimum experimental designs. Oxford University Press, USA
Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ (1984) A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 15(3):217–227. doi:10.1002/ana.410150302
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27(9):1533–1539. doi:10.1038/sj.jcbfm.9600493
Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN (2010) Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab 30(1):46–50. doi:10.1038/jcbfm.2009.190
Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49(7):538–544
Kapur S, Barsoum SC, Seeman P (2000) Dopamine D(2) receptor blockade by haloperidol. (3)H-raclopride reveals much higher occupancy than EEDQ. Neuropsychopharmacology 23(5):595–598. doi:10.1016/S0893-133X(00)00139-1
Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M (2005) Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 115(3):254–263. doi:10.1016/j.pain.2005.02.032
Dodds MG, Hooker AC, Vicini P (2005) Robust population pharmacokinetic experiment design. J Pharmacokinet Pharmacodyn 32(1):33–64. doi:10.1007/s10928-005-2102-z
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10928_2015_9410_MOESM1_ESM.tiff
Supplementary material 1 (TIFF 16406 kb). The distribution of the MED for each of the models when they were selected using the likelihood ratio test. The dashed line indicates the true MED. The scale is truncated such that all doses above 4 are set to 4. The cases when no significant effect of a linear versus a no effect model was seen are shown at a dose of 4
Rights and permissions
About this article
Cite this article
Kågedal, M., Karlsson, M.O. & Hooker, A.C. Improved precision of exposure–response relationships by optimal dose-selection. Examples from studies of receptor occupancy using PET and dose finding for neuropathic pain treatment. J Pharmacokinet Pharmacodyn 42, 211–224 (2015). https://doi.org/10.1007/s10928-015-9410-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9410-8