Abstract
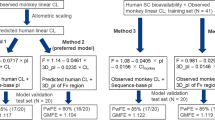
Predicting the pharmacokinetic (PK) time course of a subcutaneously (SC) administered novel therapeutic protein using in silico approaches offers an opportunity to streamline the drug development process by facilitating selection of starting and target doses in initial human trials. Herein, we propose a workflow for predicting the human exposure time course following SC administration. Leveraging knowledge obtained following both intravenous and SC administration in monkeys, this workflow employs the development of a whole body physiologically-based pharmacokinetic (PBPK) model incorporating vascular circulation, lymphatic uptake and both renal and non-specific clearance mechanisms to predict the PK of a novel pegylated peptide. Optimization of the model was initially performed in monkeys, after which the model was scaled up to human proportion. Inclusion of a SC depot compartment allowed for precise simulation of the SC time course in monkeys. Simulated human exposure after SC administration was within approximately 20 % of the observed values and successfully predicted the time course of two subsequent dosing levels. This workflow represents one of the first publications of a PBPK workflow to predict the time course of a SC administered therapeutic protein based off of a single, non-human primate species and shows promise in facilitating the dose selection in first-in-human dose escalation studies for novel protein therapeutics.







Similar content being viewed by others
References
Offman E, Edginton AN (2013) Contrasting toxicokinetic evaluations and interspecies pharmacokinetic scaling approaches for small molecules and biologics: applicability to biosimilar development. Xenobiotica 43:561–569
Ling J, Zhou H, Jiao Q, Davis HM (2009) Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol 49:1382–1402
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S (2011) Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? Ther Monoclon Antib 3:61–66
Supersaxo A, Hein W, Gallati H, Steffen H (1988) Recombinant human interferon alpha-2a: delivery to lymphoid tissue by selected modes of application. Pharm Res 5:472–476
Supersaxo A, Hein WR, Steffen H (1990) Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res 7:167–169
Dahlberg AM, Kaminskas LM, Smith A, Nicolazzo JA, Porter CJ, Bulitta JB, McIntosh MP (2014) The lymphatic system plays a major role in the intravenous and subcutaneous pharmacokinetics of trastuzumab in rats. Mol Pharm 11:496–504
Wang W, Chen N, Shen X, Cunningham P, Fauty S, Michel K, Wang B, Hong X, Adreani C, Nunes CN, Johnson CV, Yin KC, Groff M, Zou Y, Liu L, Hamuro L, Prueksaritanont T (2012) Lymphatic transport and catabolism of therapeutic proteins after subcutaneous administration to rats and dogs. Drug Metab Dispos 40:952–962
Richter WF, Bhansali SG, Morris ME (2012) Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J 14:559–570
Rippe B, Haraldsson B (1994) Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 74:163–219
Rippe B, Haraldsson B (1987) Fluid and protein fluxes across small and large pores in the microvasculature. Application of two-pore equations. Acta Physiol Scand 131:411–428
Zhao L, Ji P, Li Z, Roy P, Sahajwalla CG (2013) The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J Clin Pharmacol 53:314–325
Kagan L, Mager DE (2013) Mechanisms of subcutaneous absorption of rituximab in rats. Drug Metab Dispos 41:248–255
Zheng Y, Tesar DB, Benincosa L, Birnbock H, Boswell CA, Bumbaca D, Cowan KJ, Danilenko DM, Daugherty AL, Fielder PJ, Grimm HP, Joshi A, Justies N, Kolaitis G, Lewin-Koh N, Li J, McVay S, O’Mahony J, Otteneder M, Pantze M, Putnam WS, Qiu ZJ, Ruppel J, Singer T, Stauch O, Theil FP, Visich J, Yang J, Ying Y, Khawli LA, Richter WF (2012) Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs 4:243–255
Shah D, Betts A (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39:67–86
Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH (2008) A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol 8:401–413
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK (1995) Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res 55:4611–4622
Ferl GZ, Wu AM, DiStefano JJ III (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33:1640–1652
R Core Team (2013) R: A language and environment for statistical computing
Graf JF, Scholz BJ, Zavodszky MI (2012) BioDMET: a physiologically based pharmacokinetic simulation tool for assessing proposed solutions to complex biological problems. J Pharmacokinet Pharmacodyn 39:37–54
Kawai R, Mathew D, Tanaka C, Rowland M (1998) Physiologically based pharmacokinetics of cyclosporine A: extension to tissue distribution kinetics in rats and scale-up to human. J Pharmacol Exp Ther 287:457–468
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Swartz MA (2001) The physiology of the lymphatic system. Adv Drug Deliv Rev 50:3–20
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709
Baumann A, Tuerck D, Prabhu S, Dickmann L, Sims J (2014) Pharmacokinetics, metabolism and distribution of PEGs and PEGylated proteins: quo vadis? Drug Discov Today 19:1623–1631
Woodburn KW, Fong KL, Wilson SD, Sloneker S, Strzemienski P, Solon E, Moriya Y, Tagawa Y (2013) Peginesatide clearance, distribution, metabolism, and excretion in monkeys following intravenous administration. Drug Metab Dispos 41:774–784
Iwama R, Sato T, Sakurai K, Takasuna K, Ichijo T, Furuhama K, Satoh H (2014) Estimation of glomerular filtration rate in cynomolgus monkeys (Macaca fascicularis). J Vet Med Sci 76:1423–1426
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, Hurh E, Gibbs MA (2011) Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet 50:131–142
Wang W, Prueksaritanont T (2010) Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos 31:253–263
Li Z, Rana TM (2014) Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 13:622–638
Caliceti P, Veronese FM (2003) Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev 55:1261–1277
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:14
Kusterle M, Jevsevar S, Porekar VG (2008) Size of pegylated protein conjugates studied by various methods. Acta Chim Slov 55:594–601
Bacher G, Szymanski WW, Kaufman SL, Zollner P, Blaas D, Allmaier G (2001) Charge-reduced nano electrospray ionization combined with differential mobility analysis of peptides, proteins, glycoproteins, noncovalent protein complexes and viruses. J Mass Spectrom 36:1038–1052
Pease LF III, Elliott JT, Tsai DH, Zachariah MR, Tarlov MJ (2008) Determination of protein aggregation with differential mobility analysis: application to IgG antibody. Biotechnol Bioeng 101:1214–1222
Woo S, Jusko WJ (2007) Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos 35:1672–1678
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Conflict of interest
No industry funds were received for the novel modeling and simulation aspects of this research. The raw concentration data was gifted to the authors by a party wishing to remain anonymous with written permission to use the data for in silico modeling purposes and publication purposes. EO is a PhD candidate at the School of Pharmacy, University of Waterloo and a paid employee of Celerion, a clinical contract research organization specializing in early human drug development.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
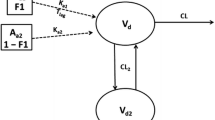
Appendix 1: representative mass balance equations for a generic PBPK model for SC administration of a pegylated protein conjugate with renal and non-specific clearance
Venous plasma circulation
Arterial plasma circulation
Lymph
Lung vascular
Lung interstitial
Kidney vascular
Kidney interstitial
Skin vascular
Skin interstitial
Skin subcutaneous depot
Liver vascular
Liver interstitial
Remaining organs vascular
Remaining organs interstitial
- FGFR:
-
Fraction of glomerular filtration rate attributed to renal clearance
- GFR:
-
Glomerular filtration rate
- ISF (subscripted):
-
Interstitial space
- L:
-
Lymph flow
- NRCL:
-
Non-renal clearance
- Pla (subscripted):
-
Plasma
- Q:
-
Blood Flow
- Vart, Vven :
-
Volume of the arterial and venous plasma space
- Vlymph :
-
Volume of the lymph node space
- Visf,organ :
-
Volume of the organ interstitial space
- Vpla,organ :
-
Volume of the organ plasma vascular space
- σv,sf :
-
Vascular reflection coefficient, scaling factor
- σv,organ :
-
Organ vascular reflection coefficient
- σv,isf :
-
Interstitial reflection coefficient
Appendix 2
See Table 4 .
Rights and permissions
About this article
Cite this article
Offman, E., Edginton, A.N. A PBPK workflow for first-in-human dose selection of a subcutaneously administered pegylated peptide. J Pharmacokinet Pharmacodyn 42, 135–150 (2015). https://doi.org/10.1007/s10928-015-9406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9406-4