Abstract
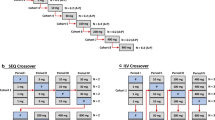
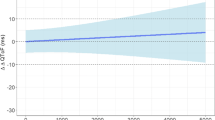
Monte Carlo simulation was used to assess the performance of linear mixed effect models (LMEMs) to characterize the concentration–effect relationship when both parent and metabolite concentrations were available. Parent and metabolite concentrations were simulated under an experimental design that mimicked a thorough QT study. Simulations were done where both parent and metabolite concentration prolonged double-delta time-matched placebo controlled QTc (ddQTc) intervals. LMEMs were then estimated where parent and metabolite concentrations, parent concentrations alone, or metabolite concentrations alone were used as the independent variables. The relative error in the estimation of the parent and metabolite slope compared to known theoretical values was calculated. Additional simulations were done comparing the estimate of the one-sided upper 95 % confidence interval at 1 time unit post-dose in an under- and overfit model compared to the true data generating model. When both parent and metabolite were included in the LMEM and both parent and metabolite prolonged ddQTc intervals, despite some high correlations between parent and metabolite concentrations, under all conditions the parent slope parameter θ p was estimated with good accuracy, having a mean relative error within ±20 % of the true value, whereas estimation of the metabolite slope θ m was overpredicted with bias increasing as the ratio of the metabolite elimination rate constant (K elm ) to metabolite formation rate constant (K f ) increased. Variability in θ m relative error increased as the metabolite potency decreased. Metabolite ratio had no effect on the ability to estimate the parent or metabolite slope. When only parent or metabolite concentration was used as the independent variable in a LMEM and both parent and metabolite concentrations prolonged ddQTc intervals, severe omitted variable bias resulted. When estimating the one-sided upper 95 % confidence interval, underfitted models tended to have higher predicted values which would suggest that an underfitted model would be more likely to declare a “QT effect”, whereas an overfitted model resulted in little difference compared to the true model. When time-matched parent and metabolite concentrations are available, both parent and metabolite concentrations should be included in the model simultaneously recognizing that the estimation of the metabolite slope may be biased under certain conditions. It is better to have an overfitted model than an underfitted model, although model simplification through removal of nonsignificant model terms in an overfitted model should not be neglected. Including both parent and metabolite concentrations in a model, even when the metabolite does not affect ddQTc intervals, does not tend to result in biased parameter estimates and in most cases will not result in inflated confidence intervals for predicted values, e.g., around maximal concentrations at the therapeutic dose. It is recommended that when time-matched parent and metabolite concentrations are available, parent and metabolite concentrations not be analyzed separately because either one or the other will result in biased parameter estimates and inflated point estimates and confidence intervals for predicted values.







Similar content being viewed by others
References
United States Department of Health and Human Services, Food and Drug Administration, and Center for Drug Evaluation and Research (2005) Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (E14). http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129357.pdf. Accessed 29 Dec 2012
Bonate PL (2001) Assessment of QTc interval prolongation in a Phase I study using Monte Carlo simulation. In: Presented at the 9th workshop on “Advanced Methods of Pharmacokinetic & Pharmacodynamic Systems Analysis”, Marina del Rey, CA
Bonate PL (2003) Assessment of QTc interval prolongation in a Phase I study using Monte Carlo simulation. In: Simulation for designing clinical trials: a pharmacokinetic-pharmacodynamic modeling perspective. Marcel Dekker, New York
Shi J, Ludden TM, Melikian AP, Gastonguay MR, Hinderling PH (2001) Population pharmacokinetics and pharmacodynamics of sotalol in pediatric patients with supraventricular or ventricular tachyarrhythmia. J Pharmacokinet Pharmacodyn 28:555–575
Garnett CE et al (2008) Concentration–QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 47:13–18
Malhotra BK, Glue P, Sweeney KR, Anziano R, Mancuso J, Wicker P (2007) Thorough QT study with recommended and supratherapeutic doses of tolterodine. Clin Pharmacol Ther 81:377–385
Chen D, Lai R, Zomorodi K, Atluri H, Ho J, Luo W, Tovera J, Bonzo D, Cundy K (2012) Evaluation of gabapentin enacarbil on cardiac repolarization: a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther 34:351–362
Carlson GF, Tou CKP, Parikh S, Birmingham BK, Butler K (2011) Evaluation of the effect of dapagliflozin on cardiac repolarization: a thorough QT/QTc study. Diabetes Ther 2:123–132
Zhu H, Wang Y, Gobburu JV, Garnett CE (2010) Considerations for clinical trial design and data analyses of thorough QT studies using drug–drug interactions. J Clin Pharmacol 50:1106–1111
Mansbach RS, Ludington E, Rogowski R, Kittrelle JP, Jochelson P (2011) A placebo- and active-controlled assessment of 6- and 50-mg oral doxepin on cardiac repolarization in healthy volunteers: a thorough QT evaluation. Clin Ther 33:851–862
Anzemet (dolasetron mesylate) package insert. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3df5f9f0-d374-425f-8a6d-e58fbfb8ea04. Accessed 12 Dec 2012
Bonate PL (1999) The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res 16:709–717
Ribbing J, Jonsson EN (2004) Power, selection bias, and predictive performance of the population pharmacokinetic covariate model. J Pharmacokinet Pharmacodyn 31:109–134
Carroll RJ, Ruppert D, Stefanski LA (1995) Measurement error in nonlinear models. Chapman and Hall, New York
Greene WH (2011) Econometric analysis, 7th edn. Prentice-Hall, Upper Saddle River
Hunyh C-L (2000) Extraneous variables and the interpretation of regression coefficients. Mult Linear Regres Viewp 26:28–35
Acknowledgments
The author would like the thank the reviewers for their thoughtful comments. I would also like to especially thank Bernard Sebastian, Barry Koplowitz, Georg Ferber, and Yaning Wang for their comments and encouragement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonate, P.L. The effects of active metabolites on parameter estimation in linear mixed effect models of concentration–QT analyses. J Pharmacokinet Pharmacodyn 40, 101–115 (2013). https://doi.org/10.1007/s10928-012-9292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9292-y