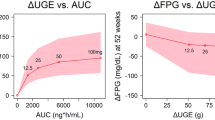
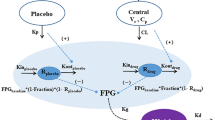
Abstract
Selecting dosing regimens for phase 2 studies for a novel glucokinase activator LY2599506 is challenging due to the difficulty in modeling and assessing hypoglycemia risk. A semi-mechanistic integrated glucose-insulin-glucagon (GIG) model was developed in NONMEM based on pharmacokinetic, glucose, insulin, glucagon, and meal data obtained from a multiple ascending dose study in patients with Type 2 diabetes mellitus treated with LY2599506 for up to 26 days. The series of differential equations from the NONMEM model was translated into an R script to prospectively predict 24-h glucose profiles following LY2599506 treatment for 3 months for a variety of doses and dosing regimens. The reduction in hemoglobin A1c (HbA1c) at the end of the 3-month treatment was estimated using a transit compartment model based on the simulated fasting glucose values. Two randomized phase 2 studies, one with fixed dosing and the other employing conditional dose titration were conducted. The simulation suggested that (1) Comparable HbA1c lowering with lower hypoglycemia risk occurs with titration compared to fixed-dosing; and (2) A dose range of 50–400 mg BID provides either greater efficacy or lower hypoglycemia incidence or both than glyburide. The predictions were in reasonable agreement with the observed clinical data. The model predicted HbA1c reduction and hypoglycemia risk provided the basis for the decision to focus on the dose-titration trial and for the selection of doses for the demonstration of superiority of LY2599506 to glyburide. The integrated GIG model represented a valuable tool for the evaluation of hypoglycemia incidence.








Similar content being viewed by others
Abbreviations
- ka:
-
Absorption rate constant
- CL/F:
-
Apparent clearance
- V/F:
-
Apparent volume of distribution
- BMI:
-
Body mass index
- CFB:
-
Change from baseline
- FPG:
-
Fasting plasma glucose
- GIG:
-
Glucose-insulin-glucagon
- GK:
-
Glucokinase
- GKA:
-
Glucokinase activator
- HbA1c:
-
Hemoglobin A1c
- LOCF:
-
Last observation carried forward
- LC–MS/MS:
-
Liquid chromatography/tandem mass spectrometry
- MAD:
-
Multiple ascending dose
- M&S:
-
Modeling and simulation
- PopPK:
-
Population PK
- PPG:
-
Postprandial glucose
- RBC:
-
Red blood cell
- SMBG:
-
Self-monitored blood glucose
- SUs:
-
Sulfonylureas
- T2DM:
-
Type 2 diabetes mellitus
References
Matschinsky FM (2005) Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep 5(3):171–176
Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206(Pt 12):2049–2057
Iynedjian PB (2009) Molecular physiology of mammalian glucokinase. Cell Mol Life Sci 66(1):27–42
Kevin R, Guertin JG (2006) Small molecule glucokinase activators as glucose lowering agents: a new paradigm for diabetes therapy. Curr Med Chem 13(15):1839–1843
Grimsby J, Berthel SJ, Sarabu R (2008) Glucokinase activators for the potential treatment of type 2 diabetes. Curr Top Med Chem 8(17):1524–1532
Matschinsky FM, Porte D (2010) Glucokinase activators (GKAs) promise a new pharmacotherapy for diabetics. F1000 Med Rep 2:43
Bue-Valleskey JM, Schneck KB, Sinha VP, Wondmagegnehu ET, Kapitza C, Miller JW (2011) LY2599506, a novel glucokinase activator (GKA), improves fasting and postprandial glucose in patients with type 2 diabetes mellitus (T2DM). Diabetes 60(Suppl 1):A272
Sheiner LB, Steimer JL (2000) Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol 40:67–95
Karlsson MO, Anehall T, Friberg LE, Henningsson A, Kloft C, Sandstrom M, Xie R (2005) Pharmacokinetic/pharmacodynamic modelling in oncological drug development. Basic Clin Pharmacol Toxicol 96(3):206–211
Allerheiligen SR (2010) Next-generation model-based drug discovery and development: quantitative and systems pharmacology. Clin Pharmacol Ther 88(1):135–137
Zhang L, Pfister M, Meibohm B (2008) Concepts and challenges in quantitative pharmacology and model-based drug development. AAPS J 10(4):552–559
Holford N, Ma SC, Ploeger BA (2010) Clinical trial simulation: a review. Clin Pharmacol Ther 88(2):166–182
Chien JY, Friedrich S, Heathman MA, de Alwis DP, Sinha V (2005) Pharmacokinetics/pharmacodynamics and the stages of drug development: role of modeling and simulation. AAPS J 7(3):E544–E559
Dahl SG, Aarons L, Gundert-Remy U, Karlsson MO, Schneider YJ, Steimer JL, Troconiz IF (2010) Incorporating physiological and biochemical mechanisms into pharmacokinetic-pharmacodynamic models: a conceptual framework. Basic Clin Pharmacol Toxicol 106(1):2–12
Jauslin PM, Silber HE, Frey N, Gieschke R, Simonsson US, Jorga K, Karlsson MO (2007) An integrated glucose-insulin model to describe oral glucose tolerance test data in type 2 diabetics. J Clin Pharmacol 47(10):1244–1255
Silber HE, Jauslin PM, Frey N, Karlsson MO (2010) An integrated model for the glucose-insulin system. Basic Clin Pharmacol Toxicol 106(3):189–194
Jauslin PM, Frey N, Karlsson MO (2011) Modeling of 24-hour glucose and insulin profiles of patients with type 2 diabetes. J Clin Pharmacol 51(2):153–164
Schneck KB, Zhang X, Bauer R, Karlsson MO, Sinha VP (2012) Assessment of glycemic response to an oral glucokinase activator in a proof of concept study: application of a semi-mechanistic, integrated glucose-insulin-glucagon model. J Pharmacokinet Pharmacodyn. doi:10.1007/s10928-012-9287-8
Hamren B, Bjork E, Sunzel M, Karlsson M (2008) Models for plasma glucose, HbA1c, and hemoglobin interrelationships in patients with type 2 diabetes following tesaglitazar treatment. Clin Pharmacol Ther 84(2):228–235
Garber AJ, Donovan DS Jr, Dandona P, Bruce S, Park JS (2003) Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab 88(8):3598–3604
Marbury T, Huang WC, Strange P, Lebovitz H (1999) Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract 43(3):155–166
Wolffenbuttel BH, Landgraf R (1999) A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Dutch and German Repaglinide Study Group. Diabetes Care 22(3):463–467
Dills DG, Schneider J (1996) Clinical evaluation of glimepiride versus glyburide in NIDDM in a double-blind comparative study. Glimepiride/Glyburide Research Group. Horm Metab Res 28(9):426–429. doi:10.1055/s-2007-979831
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352(9131):837–853
Lesko LJ, Rowland M, Peck CC, Blaschke TF (2000) Optimizing the science of drug development: opportunities for better candidate selection and accelerated evaluation in humans. Pharm Res 17(11):1335–1344
Galluppi GR, Rogge MC, Roskos LK, Lesko LJ, Green MD, Feigal DW Jr, Peck CC (2001) Integration of pharmacokinetic and pharmacodynamic studies in the discovery, development, and review of protein therapeutic agents: a conference report. Clin Pharmacol Ther 69(6):387–399
Bonate PL (2000) Clinical trial simulation in drug development. Pharm Res 17(3):252–256
Kimko HC, Peck CC (eds) (2011) Clinical trial simulation: application and trends. AAPS advances in the pharmaceutical sciences series, vol 1. Springer-Verlag, New York
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16(1):187–196
Hasegawa M, Imai Y, Hiraoka M, Ito K, Roy A (2011) Model-based determination of abatacept exposure in support of the recommended dose for Japanese rheumatoid arthritis patients. J Pharmacokinet Pharmacodyn 38(6):803–832
Marchand M, Fuseau E, Critchley DJ (2010) Supporting the recommended paediatric dosing regimen for rufinamide in Lennox-Gastaut syndrome using clinical trial simulation. J Pharmacokinet Pharmacodyn 37(1):99–118
de Greef R, Zandvliet AS, de Haan AF, Ijzerman-Boon PC, Marintcheva-Petrova M, Mannaerts BM (2010) Dose selection of corifollitropin alfa by modeling and simulation in controlled ovarian stimulation. Clin Pharmacol Ther 88(1):79–87
Kimko HC, Reele SS, Holford NH, Peck CC (2000) Prediction of the outcome of a phase 3 clinical trial of an antischizophrenic agent (quetiapine fumarate) by simulation with a population pharmacokinetic and pharmacodynamic model. Clin Pharmacol Ther 68(5):568–577
Matschinsky FM, Zelent B, Doliba N, Li C, Vanderkooi JM, Naji A, Sarabu R, Grimsby J (2011) Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care 34(Suppl 2):S236–S243
Kimko H, Duffull SB (2003) Simulation for designing clinical trials: a pharmacokinetic-pharmacodynamic modeling perspective drugs and the pharmaceutical sciences. Marcel Dekker, New York
Hoffman R (ed) (2005) Hematology: basic principles and practice. Elsevier Churchill Livingston, Philadelphia
Bergman RN, Ider YZ, Bowden CR, Cobelli C (1979) Quantitative estimation of insulin sensitivity. Am J Physiol 236(6):E667–E677
Cherrington AD (1999) Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 48(5):1198–1214
Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD (2011) Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 13(Suppl 1):118–125
Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P (2011) Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab 13(Suppl 1):95–105
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ (2008) Translating the A1C assay into estimated average glucose values. Diabetes Care 31:1473–1478
Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE (2002) Defining the relationship between plasma glucose and HbA1c. Diabetes Care 25:275–278
Acknowledgments
The authors thank the entire Lilly GKA team for making the clinical data available.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Schneck, K., Bue-Valleskey, J. et al. Dose selection using a semi-mechanistic integrated glucose-insulin-glucagon model: designing phase 2 trials for a novel oral glucokinase activator. J Pharmacokinet Pharmacodyn 40, 53–65 (2013). https://doi.org/10.1007/s10928-012-9286-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9286-9