Abstract
AZD9773 is an ovine-derived, polyclonal, anti-tumour necrosis factor-alpha (TNF-α) antibody fragment. Using data from an AZD9773 Phase IIa study in patients with severe sepsis (clinicaltrials.gov: NCT00615017), a population pharmacokinetic/pharmacodynamic (PK/PD) model was developed. The model assessed the influence of various covariates on the PK of AZD9773 and the relationship between AZD9773 exposure and serological TNF-α concentration. A linear two-compartment model was used to describe AZD9773 concentration-time data. A stepwise covariate analysis was performed on the PK parameters. Subsequently, the serological TNF-α concentrations and drug effect were captured using an indirect response model, with a variable production rate of TNF-α described by a quadratic function. Creatinine clearance (CrCL) was the only covariate with a significant effect on the PK of AZD9773. A typical patient’s drug clearance varied with CrCL; the relationship was non-linear. Diagnostic analysis of the PK/PD model showed that the fit was good, both across cohorts and in AZD9773-treated versus placebo patients. Serological TNF-α concentrations and the reduction of measurable serum TNF-α by AZD9773 were well characterized across all the cohorts evaluated in the Phase IIa study. This population PK/PD model was subsequently used to simulate alternative dosing options for a Phase IIb study (clinicaltrials.gov: NCT01145560).
Similar content being viewed by others
Introduction
Sepsis is the primary cause of death in non-cardiac intensive care units (ICUs) and leads to substantially decreased quality of life in patients who survive [1, 2]. Current management of sepsis includes eradication of the infection source and/or use of appropriate antimicrobial treatment, together with aggressive supportive care such as fluid resuscitation and restoration of tissue perfusion using inotropes, vasopressors and/or blood transfusions, or pulmonary therapy using supplemental oxygen and mechanical ventilation [3, 4]. The release of cytokines into the circulation is key in the initial development and perpetuation of sepsis [5]. Animal septicaemia models have demonstrated that the pro-inflammatory cytokine tumour necrosis factor-alpha (TNF-α) plays a significant role in activating the cytokine cascade [6–8]. TNF-α is one of the first ‘proximal’ cytokines to be released by macrophages in response to infection, stimulating the production of later ‘distal’ cytokines such as interleukin-6 (IL-6) and IL-8, which intensify and propagate the inflammatory response [5]. As an early mediator of the inflammatory response, TNF-α may be an effective target in the treatment of sepsis.
Although the interest in immunomodulatory treatment has grown in recent years, efficacy has been limited to date [9]. Drotrecogin alfa (activated) recombinant human (rh) protein C (Xigris®; Eli Lilly and Company, Indianapolis, IN, USA) was, until recently, the only immunomodulatory drug specifically approved for the treatment of severe sepsis. However, the drug was withdrawn in October 2011 following the results of the placebo-controlled PROWESS-SHOCK study, where the primary endpoint of a statistically significant reduction in 28-day all-cause mortality was not met. AZD9773 is a preparation of polyclonal antibody fragments (Fabs) generated in sheep immunized with rhTNF-α. CytoFab, an earlier development formulation of AZD9773, reduced plasma TNF-α and significantly increased the number of ventilator- and ICU-free days in patients with severe sepsis compared with placebo (15.0 vs 9.8, P = 0.04; 12.6 vs 7.6, P = 0.03, respectively) [10]. In addition, all-cause 28-day mortality rates were reduced in the CytoFab group compared with placebo (26 vs 37 %; P = 0.274) [10].
In a Phase IIa study in patients with severe sepsis (clinicaltrials.gov: NCT00615017), the safety and tolerability profiles of intravenous single and multiple doses of AZD9773 were characterized; assessments of the pharmacokinetic (PK) and pharmacodynamic (PD) profiles (the effect on TNF-α serum concentrations) were also performed. AZD9773 was well tolerated and rapidly (within 2 h) decreased serological TNF-α concentrations to near-undetectable levels. Using the data generated from this Phase IIa study, we developed a population PK/PD model assessing the influence of various covariates on the PK of AZD9773, as well as the relationship between AZD9773 exposure and TNF-α; the aim was to support further trial design. There is little precedent for PK/PD modelling of TNF-α antagonists beyond PK summaries and analysis of outcome; we therefore believe that this is the first analysis linking drug exposure to a reduction in circulating TNF-α concentrations in the clinic.
Methods
Phase IIa study design and sample collection
In the double-blind, placebo-controlled, dose-escalation Phase IIa study, the safety, efficacy, PK and PD profiles of single and multiple intravenous infusions of AZD9773 were investigated in patients with severe sepsis [11]; this study was not powered to assess efficacy endpoints such as overall survival. Patients were sequentially recruited into one of five cohorts and then randomized 2:1 to receive either AZD9773 or placebo (Table 1). The study methods have been published in more detail elsewhere [11].
Blood samples for PK assessment were collected pre-infusion, at the end of the infusion and at 0.5, 1, 2, 4, 8, 12, 24, 48 and 72 h post-infusion for cohorts 1 and 2. Samples for cohorts 3–5 were collected pre-infusion for the loading dose and maintenance doses 5, 7 and 9 (i.e. the last one), and at the end of the infusion and at 0.5, 1, 2, 4, 8 and 12 h post-infusion for the loading dose and last maintenance dose.
Bioanalytical methodology for the detection of AZD9773 Fab fragments and TNF-α
AZD9773 is a mixture of ovine anti-human TNF-α (specific) and total Fabs; total concentrations are considered here. The total ovine Fabs (both unbound and bound to circulating TNF-α) were quantitatively determined in serum by direct ELISA assays (the limit of quantification was 5 ng/mL; Quotient Bioresearch, Cambridge, UK). The ELISA assay utilized a rabbit anti-sheep Fab fragment as the capture antibody, with a horseradish peroxidase (HRP)-conjugated rabbit antisheep IgG Fab specific antibody to detect the total AZD9773 Fab fragments in serum. The calibration range was 20–80 ng/mL for the total Fab ELISA.
Blood samples for evaluation of TNF-α concentration were drawn pre-infusion, 2 and 3 h after the initial infusion ended, and 24, 48, 72, 96, 120, 144 and 168 h after start of infusion. Serological TNF-α concentrations were measured using the Quantikine® high-sensitivity enzyme immunoassay (R&D Systems, Minneapolis, MN, USA); the limit of quantification was 1.3 pg/mL [12].
Population PK/PD model
The primary objective of this analysis was to use data from the Phase IIa study to develop a population model describing the plasma PK profile of AZD9773 and the corresponding inter- and intra-patient variability following intravenous drug administration, including evaluation of the influence of various covariates on the population PK parameters. The secondary objectives were to predict Bayesian individual exposure of AZD9773 and to use these data to perform exposure-response modelling analysis of serological TNF-α concentration.
PK and PD analyses
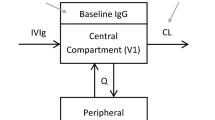
Serum PK of total AZD9773 was described by a linear two-compartment model (Fig. 1) parameterized in terms of clearances and volumes.
where A 1 and A 2 are the amounts of drug in the two compartments and C p is the predicted serum concentration of AZD9773. All other parameters are defined in Table 2.
The development of the base model (inter-individual variability, but no covariate effects) was followed by the addition of relevant covariates to the identified structural model parameters. The covariate model was developed based on a comprehensive stepwise-forward selection and stepwise-backward elimination procedure for the 12 specified covariates: age, weight, body mass index (BMI), sex, race, serum creatinine, serum albumin, serum bilirubin, sequential organ failure assessment score, acute physiology and chronic health evaluation II score, dialysis and creatinine clearance (CrCL). The final full covariate models were assessed for their interpretability and goodness of fit to the observed AZD9773 concentration data. Individual empirical maximum Bayesian estimates of PK parameters were calculated based on the final AZD9773 PK model.
For a covariate to be included in the PK model after each forward selection or backward elimination step, it had to meet criteria for both statistical significance and clinical relevance. The statistically significant criterion was pre-specified as P = 0.001 (or 10.83 units of change in the minimum value of objective function from a NONMEM run, assuming a Chi-squared distribution with a degree of freedom equal to 1). All reported root mean standard errors (RSEs) were estimated using a bootstrap method with 100 iterations.
The model derived from the PK analysis step was subsequently used to provide predicted AZD9773 concentrations for fitting an indirect response model to the TNF-α concentration-time data. This models the formation of AZD9773:TNF-α complexes:
where k bind is the constant of proportionality between drug concentration and drug-mediated reduction of TNF-α concentrations. The model does not include dissociation of drug and target. This simplification minimizes the number of parameters to identify from the dataset and the model diagnostics demonstrate the model to be adequate.
The apparent appearance of TNF-α in the serum was described by a quadratic function; this was based on an initial observation that TNF-α concentrations in patients receiving placebo varied over time. As such, an indirect response model with a constant production rate was not appropriate and a time-dependent production rate was used. The functional form of this empirical production rate was chosen to be as economical as possible, using minimal parameters to describe the changes in TNF-α concentration over time. The TNF-α input rate, where ‘t’ is time from the start of the study, was therefore:
To assist with understanding the apparent rate of TNF-α appearance and the inter-individual variability, the above formula was rewritten with three new parameters: slope, describing the initial slope; peak, describing the peak (or trough) rate of appearance; and time to peak (ttp), describing the time at which this peak (or trough) was achieved. It can be then shown that:
For all structural parameters in the model, a log-normal distribution for inter-individual variability was assumed:
The exception was the parameter slope, which could feasibly be negative or positive and so was parameterized as being normally distributed:
The model was applied to the whole dataset. It was assessed for goodness of fit, including the population and individual predictions versus observed concentrations, the population predictions versus conditional weighted residuals (CWRES), and the distribution of residuals over time, across AZD9773- and placebo-treated patients.
The software packages NONMEM (version 7; ICON; Ellicott City, MD, USA) and R (version 2.8.0; The R Foundation for Statistical Computing, Vienna, Austria) were used for modelling and simulation using first-order conditional estimation methodology. R was used for data preparation, graphical analysis, model diagnostics and statistical summaries. Perl-speaks-NONMEM (PsN, version 3.4.2; Department of Pharmacy, Uppsala University, Uppsala, Sweden) was used to implement all the NONMEM runs for the population PK modelling and visual predictive check for model diagnostics. Xpose (versions 4.0–4.8; Department of Pharmacy, Uppsala University, Uppsala, Sweden) and R were used to produce all the model diagnostic plots.
Results
Patients and analysis dataset
The Phase IIa study was conducted at 27 US centres between January 2008 and July 2009. Of the 70 randomized patients who were treated, 47 received AZD9773 and 23 received placebo. In total, 618 PK and 523 PD (TNF-α) samples were collected (Table 1).
PK modelling
The evaluation of potential covariates found that CrCL, calculated by the Cockcroft-Gault equation [13], was the only covariate with a significant effect on the clearance of AZD9773 (Table 2). AZD9773 clearance and CrCL had a non-linear relationship, where increased CrCL predicted increased drug clearance, which could be summarized as CL = 0.654 × (CrCL/89.7)0.595 (40.3 % between-patient variability) (Fig. 1). Although creatinine clearance has been shown to be the only statistically significant covariate, it had little impact on the estimated values for the remaining parameters, except for reducing the estimated inter-individual variability on clearance as would be expected.
Diagnostic plots of the final PK model demonstrated a reasonable fit. Analysis of the CWRES and of individual patient data (Fig. 2) identified three patients with drug concentrations that were classed as outliers. Re-analysis of the PK data excluding these three patients concluded that these outliers did not substantially influence the parameter estimates. The prediction-corrected visual predictive check highlights that the model predicts a wider range of observed exposures (Fig. 3), though this is likely to be an effect of the limit of quantification. The model and the numerical predictive check diverge at the lower quantiles where predictions approach the lower LoQ. Although CrCL was found to be a significant covariate, there was little change in the overall goodness of fit. Given that the parameter estimates for AZD9773 clearance were not sensitive to the inclusion of these patients, the covariate model that was estimated using all patients was robust and the full dataset was included for PD analysis.
AZD9773 serum concentrations and prediction-corrected confidence intervals generated from 1,000 simulations of the final PK model for loading dose (left) and steady state (right). Placebo, single dose (cohorts 1 and 2) and multiple doses (cohorts 3–5) are shown. The dark grey region is the 95 % confidence interval of the median, the light grey areas are the confidence regions of the upper 97.5 % and lower 2.5 % bounds. The solid lines are the observed median, lower 2.5 % and upper 97.5 % quantiles. The dashed lines are the simulated quantiles
PD modelling
The maximum Bayesian estimates calculated for the individual primary PK parameters were used for PD analysis. The indirect response model was fitted to the TNF-α concentration-time data with log-normal random effects on peak, ttp and TNFout. A normally distributed random effect was fitted to the slope, allowing for time-varying concentrations of TNF-α, and the parameter kbind was fitted as a fixed effect only (Table 3).
Diagnostic plots of the model predictions compared with observed data showed that the fit was good (Fig. 4). In particular, the individual predictions fitted well with individual observed values across all cohorts and in placebo- versus AZD9773-treated patients. Importantly, the model characterizes the TNF-α production rate regardless of the treatment group to which a patient was randomized. Some patients exhibited high TNF-α concentrations at later time points, mainly those only receiving a single dose of AZD9773. This is consistent with drug washout. An inspection of the individual parameter estimates (not shown) showed no trend with cohort, including those patients with raised concentrations near the end of the time course. This gives confidence in the drug effect characterized by the model. The prediction-corrected visual predictive check results reflect this as well, with the model-predicted quantiles reflecting the wide range of responses observed in the data (Fig. 5). This is most likely being driven by the higher observations (>20 pg/mL). As most data points lie on or near the line of unity, on average the population means captured the TNF-α concentrations. An inspection of the maximum Bayesian estimates for the individual parameters versus covariates (not shown) yielded no potential useful covariates, and so a construction of a covariate model was not considered.
Serum TNF-α concentrations and prediction-corrected confidence intervals generated from 1,000 simulations of the PD model. Placebo, single dose (cohorts 1 and 2) and multiple doses (cohorts 3–5) are shown. The dark grey region is the 95 % confidence interval of the median, the light grey areas are the confidence regions of the upper 97.5 % and lower 2.5 % bounds. The solid lines are the observed median, lower 2.5 % and upper 97.5 % quantiles. The dashed lines are the simulated quantiles
Discussion
Serological TNF-α concentrations can vary widely both between patients and over time, as exhibited in Fig. 4; this could pose difficulties for modelling the data. In this PK/PD analysis of data generated from a Phase IIa study, serological TNF-α concentrations and the PD effect of AZD9773 on these were well characterized by the indirect PD model that was developed. There were no differences in individual parameter estimates observed between placebo- and AZD9773-treated patients. This is the first time that a clinical PK/PD analysis has been reported for an anti-TNF-α polyclonal Fab.
The PK profile of AZD9773 following single or multiple infusions was described by a linear two-compartment model. Of all the candidate covariates considered, only serum CrCL had a statistically significant effect on the PK profile of AZD9773. It was found that CrCL was a predictor of the population drug clearance, where increased CrCL predicted increased drug clearance and vice versa. In the initial covariate analysis, renal replacement dialysis was also found to be a statistically significant factor; however, it did not result in as large an objective function change as CrCL. Considering dialysis status as a covariate after incorporating CrCL did not result in a statistically significant change in the objective function. This demonstrated that renal status is the important factor (dialysis being an indicator of this), and that dialysis itself does not impact clearance. These findings are consistent with the decrease in drug clearance observed in patients with renal impairment. The final covariate model of AZD9773 included serum CrCL only. Given the range of serum CrCL (4.92–26.52 L/h) in the patient dataset, the effect associated with serum CrCL rates results in a range of mean predicted drug clearances of 0.16–1.70 L/h. This 10-fold range in CrCL would result in a range of equivalent magnitude in drug clearance and thus similar variability in the area under the plasma concentration-time curve for a given dosing regimen. As significant reductions in serological TNF-α concentration were observed in treated patients and considering the safety margin observed with AZD9773, this variability in clearance rates is likely to be of limited clinical relevance.
Although the PD model is somewhat simplistic, it does describe the data well. The bi-linear relationship between AZD9773, TNF-α and the rate of TNF-α reduction works despite the absence of complex disassociation in the model. It is likely that this is because the complex is cleared at least as rapidly as AZD9773, thus making disassociation an unimportant feature. A saturable ‘Emax’ type effect was similarly not required, with a greater rate of TNF-α reduction with increasing AZD9773 exposure.
The main limitation of the PK and PK/PD model applicability is the small patient sample size: 47 patients received AZD9773, of whom 30 received clinically relevant treatment (i.e. loading plus maintenance dosing regimen). The total number of patients (n = 70) used to characterize the PD profile may be considered small given the inter-individual variability observed.
Nevertheless, the population PK/PD model described here characterizes well the time-dependent disease progression in a heterogeneous disease population against a background of standard care. In addition, the relationship between serum AZD9773 concentrations and the reduction of TNF-α serum concentrations is well characterized. The developed model will therefore allow a model-based approach to the design of further clinical trials with AZD9773 to assess efficacy and link this with target suppression.
References
Perl TM, Dvorak L, Hwang T, Wenzel RP (1995) Long-term survival and function after suspected Gram-negative sepsis. JAMA 274:338–345
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Sharma VK, Dellinger RP (2006) Treatment options for severe sepsis and septic shock. Expert Rev Anti Infect Ther 4:395–403
Schuerholz T, Marx G (2008) Management of sepsis. Minerva Anestesiol 74:181–195
Blackwell TS, Christman JW (1996) Sepsis and cytokines: current status. Br J Anaesth 77:110–117
Fong Y, Tracey KJ, Moldawer LL, Hesse DG, Manogue KB, Kenney JS, Lee AT, Kuo GC, Allison AC, Lowry SF (1989) Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med 170:1627–1633
Hinshaw LB, Emerson TE Jr, Taylor FB Jr, Chang AC, Duerr M, Peer GT, Flournoy DJ, White GL, Kosanke SD, Murray CK (1992) Lethal Staphylococcus aureus-induced shock in primates: prevention of death with anti-TNF antibody. J Trauma 33:568–573
Redl H, Schlag G, Ceska M, Davies J, Buurman WA (1993) Interleukin-8 release in baboon septicemia is partially dependent on tumor necrosis factor. J Infect Dis 167:1464–1466
Antonopoulou A, Giamarellos-Bourboulis EJ (2011) Immunomodulation in sepsis: state of the art and future perspective. Immunotherapy 3:117–128
Rice TW, Wheeler AP, Morris PE, Paz HL, Russell JA, Edens TR, Bernard GR (2006) Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit Care Med 34:2271–2281
Morris PE, Zeno B, Bernard AC, Huang X, Das S, Edeki T, Simonson SG, Bernard GR (2012) A placebo-controlled, double-blind, dose-escalation study to assess the safety, tolerability, and pharmacokinetics/pharmacodynamics of single and multiple intravenous infusions of AZD9773 in patients with severe sepsis and septic shock. Crit Care 16:R31
R&D Systems Europe (2011) Quantikine. Human TNF-alpha Immunoassay. For the quantitative determination of human tumor necrosis factor alpha (TNF-alpha) concentrations in cell culture supernates, serum, and plasma. http://www.rndsystems.com/pdf/dta00c.pdf. Accessed 7 Sept 2012
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Acknowledgments
We thank Andrew Jones, PhD from Mudskipper Bioscience, who provided medical writing support funded by AstraZeneca. James WT Yates, Shampa Das and Guy Mainwaring are current employees of AstraZeneca. John Kemp is a former employee of AstraZeneca. This study was supported by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yates, J.W.T., Das, S., Mainwaring, G. et al. Population pharmacokinetic/pharmacodynamic modelling of the anti-TNF-α polyclonal fragment antibody AZD9773 in patients with severe sepsis. J Pharmacokinet Pharmacodyn 39, 591–599 (2012). https://doi.org/10.1007/s10928-012-9270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9270-4