Abstract
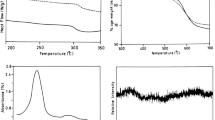
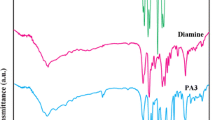
Ionic liquids (IL)s have been recognized as ‘green’ alternatives to the organic solvents in a range of synthesis, catalysis and electrochemistry due to their unique chemical and physical properties. In this investigation, a series of organosoluble, thermally stable and optically active hydroxyl-containing poly(amide–imide)s (PAI)s were prepared via polycondensation reaction of an aromatic diamine, 3,5-diamino-N-(4-hydroxyphenyl)benzamide (4), and different chiral amino acid-based diacids (3a–3e) in the presence of molten tetrabutylammonium bromide as a molten IL and triphenyl phosphite under classical heating method. This process is safe and green since toxic and volatile organic solvents such as N-methylpyrrolidone (NMP) and N,N′-dimethylacetamide (DMAc) were eliminated. The resulting new polymers were obtained in good yields with inherent viscosities ranging between 0.23 and 0.54 dL g−1 and were characterized by Fourier transform infrared spectroscopy, specific rotation, powder X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), thermogravimetric analysis, elemental analysis, and in some cases by 1H-NMR techniques. The FE-SEM micrographs and XRD showed that the synthesized PAIs were nanostructured and amorphous polymers. The effect of ultrasonic irradiation on the size of polymer particles was also investigated and the results showed that the size of polymer nanoparticles after ultrasonication became smaller than the size of them, before ultrasonic radiation. All of the polymers were readily soluble in many organic solvents such as N,N′-dimethyl sulfoxide, DMAc and NMP.








Similar content being viewed by others
References
Hamciuc E, Hamciuc C, Airinei A, Bruma M (1997) Angew Makromol Chem 245:105–112
Hsiao SH, Yang CP, Chen CW, Liou GS (2005) J Polym Res 38:627–634
Yang CP, Su YY (2005) Macromol Chem Phys 206:1947–1958
Behniafar H, Mohammadparast-delshaad S (2012) Polym Degrad Stabil 97:228–233
Welton T (1999) Chem Rev 99:2071–2083
Illescas J, Ramirez-Fuentes YS, Rivera E, Morales-Saavedra OG, Rodriguez-Rosales AA, Alzari V, Nuvoli D, Marian A (2012) J Polym Sci, Part A: Polym Chem 50:821–830
Zhang S, Feret A, Lefebvre H, Tessier M, Fradet A (2011) Chem Commun 47:11092–11094
Matsumoto K, Endo T (2011) J Polym Sci, Part A: Polym Chem 49:3582–3587
Kaneko Y, Kyutoku T, Shimomura N, Kadokawa JI (2011) Chem Lett 40:31–33
Mallakpour S, Rafiee Z (2011) Prog Polym Sci 36:1754–1765
Ye L, Ju L, Wu C, Feng T, Mo W, Wu F, Bai Y, Feng ZG (2009) J Appl Polym Sci 114:1086–1093
Bai H, Wu X, Shi G (2006) Polymer 47:1533–1537
Tsubata A, Uchiyama T, Kameyama A, Nishikubo T (1997) Macromolecules 30:5649–5654
Domanska U, Marciniak A, Krolikowski M (2008) J Phys Chem B 112:1218–1225
Mallakpour S, Taghavi M (2008) Polymer 49:3239–3249
Mallakpour S, Dinari M (2010) J Polym Environ 18:705–713
Mallakpour S, Zadehnazari A (2009) J Macromol Sci, Pure Appl Chem 46:783–789
Wulff G (2007) Angew Chem Int Ed 28:21–37
Song C, Li L, Wang F, Deng J, Yang W (2011) Polym Chem 2:2825–2829
Sogava H, Shiotsuki M, Matsuoka H, Sanda F (2011) Macromolecules 44:3338–3345
Sanda F, Yukawa Y, Masuda T (2004) Polymer 45:849–854
Mallakpour S, Zadehnazari A (2011) Exp Polym Lett 5:142–181
Maeda K, Kuroyanagi K, Sakurai SI, Yamanaka T, Yashima E (2011) Macromolecules 44:2457–2464
Liu R, Sogawa H, Shiotsuki M, Masuda T, Sanda F (2010) Polymer 51:2255–2263
In I, Kim SY (2005) Macromol Rapid Commun 206:1862–1869
Mallakpour S, Hajipour AR, Habibi S (2002) J Appl Polym Sci 86:2211–2216
Mallakpour S, Hajipour AR, Habibi S (2001) Eur Polym J 37:2435–2442
Mallakpour S, Shahmohammadi MH (2004) J Appl Polym Sci 92:951–959
Mallakpour S, Shahmohammadi MH (2005) Iran Polym J 14:473–483
Faghihi K, Foroughifar N, Mallakpour S (2004) Iran Polym J 13:93–99
Van Krevelen DW (1975) Polymer 16:615–620
Van Eldik R, Hubbard CD (1996) Chemistry under extreme or non classical conditions. Wiley, New York
Flosdorf EW, Chambers LA (1933) J Am Chem Soc 55:3051–3052
Melville HW, Murray AJR (1950) Trans Faraday Soc 46:996–1009
Acknowledgments
We wish to express our gratitude to the Research Affairs Division Isfahan University of Technology (IUT), for financial support. Further financial support from National Elite Foundation (NEF) and Center of Excellency in Sensors and Green Chemistry Research (IUT) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallakpour, S., Zadehnazari, A. Synthesis and Characterization of Novel Heat Stable and Processable Optically Active Poly(Amide–Imide) Nanostructures Bearing Hydroxyl Pendant Group in an Ionic Green Medium. J Polym Environ 21, 132–140 (2013). https://doi.org/10.1007/s10924-012-0442-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-012-0442-5