Abstract
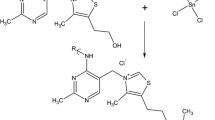
Organotin polyamine ethers containing acyclovir in their backbone were synthesized in moderate to high yield employing the aqueous interfacial polycondensation system. The products are high molecular weight polymers. Infrared spectroscopy of the products shows new bands characteristic of the formation of Sn–N and Sn–O bonds consistent with the proposed structure. MALDI-TOF MS below 2000 Da shows the presence of organotin and acyclovir units containing these two moieties. The products show moderate inhibition of a number of cancer cell lines and exhibit the ability to inhibit a number of viruses, particularly the herpes simplex virus-1 and varicella zoster virus that are responsible for herpes, chicken pox and shingles.
Similar content being viewed by others
INTRODUCTION
As an extension of our efforts to develop various antibacterial and anticancer agents we are now looking at contributions that can be made by antiviral agents. Our approach is to utilize known successful drugs and to incorporate them into polymers where the other “co-monomer” can also act to enhance the biological activity. In the present research we are coupling the biological activity of organotin moieties with the biologically active acyclovir. We are creating drugs that are active through at least two different routes lessening the potential for the microorganism to mutate to non-susceptible forms and increasing the chances that the microorganism is effectively inhibited.
Acyclovir (1), 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one, is also known as acycloguanosine and by its tradename Zovirax (TM), it is sold by Glaxo Welcome as a powder, pill, and ointment. The synthesis of acyclovir was first reported in 1974 by the Welcome Research Laboratories [1, 2]. Acyclovir, 9-(2-2-hydroxyethoxy-methyl)guanine was initially called acycloguanosine because the sugar ring of guanosine was opened up.

Acyclovir is widely used to inhibit several herpes viruses, particularly HSV-1 and HSV-2 [3]. It is also used to treat varicella zoster virus (VZV), Epstein–Barr virus (EBV), and cytomegalovirus (CMV). The inhibitory activity of acyclovir is highly selective. Thus, acyclovir is a first line antiviral drug.
Recently we reported the synthesis of the analogous titanocene, zirconocene, and hafnocene polymers (2) from reaction of the metallocene dichlorides with acyclovir [4].

Several other researchers have also made modifications to acyclovir. This includes the formation of polymeric lattice structures. For instance, Garcia-Raso et al. [5] reacted acyclovir with a variety of metal ions to form monomeric (with Ni+2, Co+2) and polymeric (Cd+2) lattice products. Turel et al. [6] formed similar products from the reaction of guanosine-5′-monophosphate (acyclovir monophosphate) with Cu+2 forming a polymeric crystal lattice.
The topic of organotin polymers has been recently reviewed [7] as has their use as anticancer agents [8].
Acyclovir contains two functional groups that are active in typical Lewis acid–base reactions. These functional groups are one alcohol and one amine. We have previously reported the synthesis of a number of organotin polymers derived from reaction with diamines and diols [9–14]. Here we report the synthesis of organotin products (3) containing acyclovir as well as initial tests for these polymers as anticancer and antiviral agents.

EXPERIMENTAL
Physical and Synthesis
Chemicals were used as received. Acyclovir (CA# 59277-89-3) was obtained from Welcome Research as a gift. Dimethyltin dichloride (CA# 753-73-1) and diphenyltin dichloride (CA# 1135-99-5) were purchased from the Aldrich Chemical Company, Milwaukee, WS. Diethyltin dichloride (CA# 866-55-7) and dibutyltin dichloride (CA# 683-18-1) were obtained from the Peninsular Chemical Research, Gainesville, FL. Dioctyltin dichloride (3542-36-7) was obtained from Alfin Chemical Company, Ward Park, MA and dicyclohexyltin dibromide (CA# 3342-69-6) was obtained from Ventron Chemicals.
The polymers were synthesized employing the classical interfacial technique. Briefly, solutions containing the Lewis base (3.00 mmol of acyclovir with 6.00 mmol of sodium hydroxide contained in 30 ml water) and Lewis acid (3.00 mmol of organotin dihalide in 30 ml hexane) were added with rapid stirring (about 18,000 rpm no load). The reaction is fast (< 15 s) and a white solid precipitates from the reaction mixture. The precipitate was recovered using vacuum filtration and washed several times with deionized water and heptane to remove unreacted materials and unwanted by-products. The solid was washed onto a glass Petri dish and allowed to dry at room temperature.
Infrared spectral studies were done utilizing a Mattson Instruments Galaxy 4020 FTIR employing potassium bromide pellets. All spectra were recorded at an instrument resolution of 4 cm−1 using 32 scans. Mass spectra were obtained utilizing a HP Mdl. G2025A MALDI-TOF Mass Spectrophotometer.
Light scattering was carried out employing a Brice-Phoenix BP 3000 Universal Light Scattering Photometer in HMPA. Refractive indices were obtained using a Bausch & Lomb Abbe Model 3-L refractometer.
Biological
Virus and Cell Lines
The two viruses used in this study were the herpes simplex virus 1, strain GHSV-UL46(HSV-1 ATCC VR-1544) supported on Vero cells and VZV, strain Ellen(VSV ATCC VR-137) supported on BS-C-1 cells. Both cell lines were propagated in monolayer cultures using minimal essential medium (MEM) with Earles’ salts, supplemented with 5% fetal bovine serum (FBS). Cells were passaged at 1:2 to 1:10 dilutions according to conventional procedures using 0.05% trypsin with 0.02% EDTA.
Drug Preparation
The drugs were prepared by dissolving the dried material into 100% DMSO at a concentration of 10 μg/ml. Working stocks were then generated by diluting stocks 1–10 into MEM yielding a final drug concentration of 1 μg/ml. From these working stocks material was transferred to MEM with 5% FBS yielding the indicated final concentrations, and the media added to the cell monolayers.
Cytotoxicity Assays
Drug cytotoxicity was determined by plating cells at a concentration of 5 × 103 or 5 × 105 cells per well in MEM with 5% FBS using a 24-well or 6-well plate and incubating the plates at 37°C, 5% CO2 for approximately 24 h until the cells divided to yield 1 × 104 or 1 × 106 cells per well. At this time, the media was removed and replaced with MEM with 5% FBS and the indicated drug concentration. Cytotoxicity was observed microscopically after 96 h with the addition of trypan blue to stain nonviable cells [15]. Assays were performed in duplicate.
HSV-1 and VZV Plaque Reduction Assays
Vero or BS-C-1 cells were grown to confluency in 6-well plates in MEM with 5% FBS. The medium was removed and the cells infected with either HSV-1 or VZV at serial 10-fold dilutions ranging from 1 × 106 to 10 plaque-forming units (PFUs) per well in 250 μl of MEM. After 30 min the medium was removed and replaced with MEM with 5% FBS and the indicated drug concentration. After incubation at 37°C and 5% CO2 for 96 h the HSV-1 infected Vero cells were observed after excitation at 395 nm for fluorescent plaques [16], and the VZV infected BS-C-1 cells stained with crystal violet to observe viral plaques [17]. Assays were performed in duplicate.
RESULTS AND DISCUSSION
Synthesis and Structural Characterization
The reaction of acyclovir with organotin dihalides to form polymers is general (Table I). The solubility of the products was tested in acetone, DMSO, DMF, DMA, and HMPA but they were only soluble in HMPA and slightly soluble in DMSO. This precludes NMR spectroscopy since deutrated HMPA is not available. Even so, molecular weight was determined by light scattering photometry and is shown in Table I. The products are high molecular weight polymers.
For drug use, the polymers are initially dissolved in a suitable solvent as HMPA or DMSO and then added to water to achieve the desired concentration. (Polymer solubility needs to be in the range of 10−2 g/ml for molecular weight determination but only 10−5 g/ml for biological testing.) It is important to understand the stability of polymers in solution. Thus molecular weight was studied over a three-month period in HMPA (Table II). The diethyltin product showed good stability with chain loss of only about 25% over the three-month period. The dimethyltin product’s chain length decreased over 99% over the test period. Even so, all of the products remained polymeric over this period of time. Tests are planned where chain length will be studied in a HMPA–water mix more closely approximating chain stability in the human body.
Infrared spectral results are consistent with the presence of moieties derived from the organotin and acyclovir. Following is a brief discussion of some of the assignments. All infrared assignments are given in terms of cm−1 and are consistent with literature assignments [4, 7, 14]. Results for the product between dimethyltin dichloride and acyclovir will be described. Bands at about 3100 are assigned as arising from C–H aromatic stretching from the acyclovir. Bands at about 2715 are assigned to the C–H aliphatic stretch from the aliphatic ether arm on the acyclovir. Bands at about 2540 are assigned to C–H stretching of methyl groups on the dimethyltin moiety. The band at about 1700 is assigned as derived from the purine ring in the acyclovir. A band at about 580 is prominent in both the spectra of the product and dimethyltin dichloride and the product and not in acyclovir. It is assigned to the Sn–C asymmetric stretching. The Sn–Cl is found in the range of 315–400 below the range of the instrument used to record the spectra. In acyclovir there is a much broader band(s) from 3500 to 3170 that includes both the N–H and O–H stretching vibrations. Much of the 3500 to 3170 band(s) is missing consistent with the absence of the –OH grouping. There is a band about 3445 that is assigned to the N–H stretch.
The Sn–N band is assigned as occurring about 1100. For the product with dimethyltin dichloride this band appears at about 1120. A similar band is found for the other products. The Sn–O band is assigned to be within the range of 400–700. A new band at about 420 is assigned to be due to the presence of the Sn–O linkage. Again, a similar band is found for the other products.
Similar results are found for the other polymers. Table III contains specific band assignments for the dibutyltin dichloride and diethyltin dichloride along with the associated polymers derived from them. Thus, the linkage of the tin moiety with the acyclovir through the nitrogen and oxygen is indicated by the infrared spectral results.
Recently, we began investigating the use of MALDI-TOF mass spectroscopy as a tool to allow a better structural identification of at least some fraction of the product [18, 19]. True MALDI requires that the polymer is soluble in some low boiling solvent that allows more intimate mixing and dispersion of the sample compound with the laser sensitive matrix liquid. The present products are only soluble in HMPA, which is not considered a volatile liquid for MALDI. In the present study we ground the sample compound with the matrix liquid giving a finely dispersed mixture. The mixture was introduced to the instrument and MS obtained in the usual fashion. Since the focus is on the ion fragments formed from fragmentation of polymer chains, this technique is referred to as Fragmentation MALDI MS or simply F MALDI MS [18, 19].
The F MALDI MS of the employed matrix alpha-cyano-4-hydroxycinnamic acid shows major ion fragments at 145 (all MS assignments are in m/e = 1 and given in Daltons) minus CO2, 189 assigned to the matrix, and 212 matrix plus sodium. These ion fragments are absent (within the background) for the spectra obtained for the polymers consistent with the spectra containing ion fragments derived from the polymer.
Following are representative results for three polymers. Table IV contains the major ion fragments present in the range of 120–2000 for the product of diphenyltin dichloride and acyclovir. A number of abbreviations are employed. Briefly, these are Sn = SnPh2, A = acyclovir (minus 2 hydrogen atoms), U = one unit, 2U = two units, and C = CH2. Thus Sn–Ph is the SnPh2 unit minus one Ph.
Tin contains ten isotopes of which seven are considered significant. At higher masses, isotope matches are difficult because of the low intensities of generated ion fragments. Even so, at lower masses such isotope matches are possible. Table V contains the isotope match for the ion fragment centered about 520 and assigned to one unit plus sodium. The match is consistent with the presence of a single tin atom in the ion fragment. The sodium is derived from the matrix solvent.
Table VI contains results for the product of diethyltin dichloride and acyclovir over the mass range of 150–2000. The abbreviations are similar to those employed in Table V except Sn = SnEt2.
Table VII contains the major ion fragments for the product of dibutyltin dichloride and acyclovir. Again, abbreviations for the assignments are similar to those employed for Table V except Sn = SnBu2.
As with other studies, hetero-atomed breakages are favored and the ring system left without fragmentation.
In summary, organotin products have been synthesized from the reaction between organotin dihalides and acyclovir with bonding occurring through Sn–O– and Sn–N–.
Antiviral Studies
The biological activities of organotin compounds are well known [4]. Organotin compounds have long have been used in the coatings industry as antifouling, antibacterial, and antifungal agents. They have also been employed as agricultural and horticultural agents against fungal diseases such as early blight, down mildew, anthracnose, and leaf and pod spot on a variety of crops. They have also been developed as pharmaceuticals, anthelmintics, and disinfectants and, more recently, as anti-tumor drugs. In this paper, we describe the initial studies involving the antiviral and anticancer activities of organotin polymers derived from the antiviral agent acyclovir.
Increasing pressures to develop antivirals to treat both old diseases, such as smallpox, and new diseases, such as SARS [20] are mounting. Viral vaccination programs are coming under increased scrutiny, including the current smallpox vaccination, with concerns about the occurrence of complications in people with immunodeficiency disorders. Additionally, a new climate appears to be emerging in which the acceptance of the risks inherent to vaccines is very low, the most recent casualty of this being the rotavirus vaccine [21]. Antivirals also have an advantage in comparison to viral vaccines because they are likely to be active against a new pandemic variant, unlike viral vaccines which are generally strain specific and do not offer broad-spectrum protection.
Antivirals, however, all suffer from the problem of target specificity [22]. Viruses, for the most part, utilize cellular machinery to replicate the viral genome and produce new virus particles. In an attempt to target viral replication, the cellular processes in uninfected cells are also undesirably affected. Polymeric drugs offer the opportunity to avoid some of these effects.
It is of interest that the concentration of cells to be tested varies between whether the goal is to test antiviral activity or ability to inhibit cancer cell growth. In evaluating the ability to inhibit cancer cell growth, 104 cells are employed for evaluation while for antiviral activity one hundred times this amount, or 106 is employed. The same sized wells are employed such that 106 cells form a continuous cell monolayer across the bottom of the plate. The wells containing 104 cells have space between cells allowing for ready replication. The conditions are such that within the plates containing 106 cells replication occurs no more than once during the test period. For the plates containing 104 cells, the lower concentration encourages rapid growth, generally about seven generations, over the test period. Thus, the cells are rapidly growing in the tests employing the lesser numbers employed for cancer studies in comparison to the much slower growing cells for the viral tests. Furthermore, the amount of drug administered is the same for wells containing 106 cells as for wells containing 104 cells so that the amount of drug per cell within the 106 cell tests is dramatically less than that available for the 104 plates. Thus, concentration effects are largely responsible for most of the observed differences. Since our intent is both to test for the drugs ability to act as anticancer drugs and to act as antiviral agents, both amounts of initial cells were studied.
Finally, there is a difference in the growth inhibition (GI) values employed. For cancer studies GI50 values are normally employed as the test measure. But for antiviral activity, GI10 values are normally employed as the test measure. The results are presented as four experiments using duplicate samples in each experiment.
Each cell line was chosen to be compatible to support growth for the particular virus. The cell lines are cancer cell lines thus some indication of their ability to inhibit cell growth is also gleamed from these studies. BSC-1 cells are African green monkey kidney epithelial cells as are vero cells but from a different strain. Both are transformed to behave as cancer cells. L929 cells are transformed mouse fibroblast cells, and 143 cells are human fibroblast bone osteosarcoma cells.
Table VIII contains GI10 values for the polymers and acyclovir employing 104 cells. In general, the order of inhibition is dibutyltin > diphenyltin > diethyltin = dioctyltin > dicyclohexyltin > acyclovir.
Table IX contains comparable data except recording the GI50 values. Here the order of inhibition is dibutyltin > diphenyltin > diethyltin > dioctyltin > dicyclohexyltin > acyclovir which is essentially what was found for the data given in Table VIII. For comparison to known values, the GI50 for cisplatin, the most widely employed anticancer drug, is about 50 μg/ml when tested against L929 cells. The values given in Table VIII are comparable or less than the value for cisplatin. In past studies by us and others, the order for ability to inhibit cell growth is generally dibutyltin > diphenyltin > diethyltin where the dicyclohexyltin and dioctyltin-containing materials are largely inactive or less active [7, 8]. As seen in Tables VIII and IX, the observed trend is in line with the values obtained for most organotin compounds including polymers. Because of the much lower toxicities of the organotin compounds [7], this makes these organotin polymers candidates for additional testing as anticancer drugs.
Table X contains GI10 values except employing 106 cells. The GI values are larger in comparison to the values obtained when employing 104 cells. The order of activity with respect to concentration to achieve a GI10 value is dibutyltin > diphenyltin > diethyltin = dioctyltin > dicyclohexyltin > acyclovir which is similar to that found when employing 104 cells.
Table XI contains data for testing 106 cells focusing on the GI50.The general trend is dibutyltin > diphenyltin > diethyltin = dioctyltin > dicyclohexyltin > acyclovir, again similar to all of the other trends. Thus, for these compounds, the number of cells tested as well as the measure, be it GI10 or GI50, results in similar inhibition trends.
As seen in Tables VIII–XI, the values for the tests initially containing 106 are significantly greater than those found for tests initially containing 104 cells. As previously noted, this is primarily attributed to the lower concentration of drug per cell within the 106 cell tests.
The viruses chosen for study represent a broad range of viruses and include those where acyclovir is known to offer good inhibition, mainly the HSV. The reovirus ST3 is a RNA virus that is currently being investigated because of its ability to inhibit certain cancer cells while leaving normal cells intact [23]. It is a representative virus responsible for many respiratory and enteric infections. Generally, drugs that are capable of inhibiting one RNA virus will be effective against other RNA viruses [24]. The other viruses are DNA viruses and the activity of DNA viruses must be studied separately. Vaccinia is the vaccine strain for small pox, and along with varicella zoster, is considered one of the common viruses that might be employed in viral terror attacks. Herpes simplex is responsible for at least 45 million infections in the US yearly, or one out of five adolescents and adults. Varicella zoster is responsible for chickenpox and shingles.
Table XII contains plaque reduction values, the values most often taken for preliminary evaluation of the ability of various compounds to inhibit viral activity. Several observations are gleaned from the data. First in all cases, many of the polymers out performed acyclovir itself. The performance is even greater when compared with the amount of acyclovir present in each sample. In general, the amount of acyclovir within the polymers represents about one half of the weight of the polymer so that all of the polymers out performed acyclovir on a total possible amount of acyclovir moiety present. Second, the order of inhibition, based on the concentration needed to effect 50% inhibition is HSV-1 > VZV > Vaccinia WR > Reovirus ST3 with little inhibition found for the reovirus but outstanding inhibition found for HSV-1 and VZV viruses. Third, the order of viral growth inhibition is similar for each of the viruses and also similar with the GI values found for the associated cancer cell lines. For HSV-1 the order is dibutyltin > diethyltin > diphenyltin = dioctyltin > acyclovir > dicyclohexyltin and for VZV the trend is diethyltin > dibutyltin > dioctyltin > diphenyl > dicyclohexyltin > acyclovir. The trend with respect to VZV is the most divergent of the trends but it still has dibutyltin and diethyltin inhibiting at the lowest concentrations.
Table XIII contains similar data except for 104 cells.
The MIC values are much lower indicating that for viral infections where the number of viruses are not great, that the polymers and acyclovir itself should demonstrate good antiviral activity. The overall trend is approximately dibutyltin > diethyltin > diphenyltin > dioctyltin > acyclovir > dicyclohexyltin again similar to that found in other parts of the study.
These studies are consistent with some of the organotin polymers, namely the dibutyltin, diethyltin and diphenyltin polymers, offering superior inhibition in comparison to acyclovir. These trends are accentuated with respect to acyclovir when considering that only about half of the polymers weight is derived from acyclovir. Thus, the activities are not due to the acyclovir alone, but are enhanced either because of the presence of the organotin moiety, presence of the acyclovir within a polymer, through control release of the acyclovir, or some combination of these factors.
Little work has been done regarding organotin compounds and antiviral activity. Much of this work was directed by Ward where a number of octahedral organotin complexes of the form R2SnL2X2 where R = Et or Ph, X = Cl or Br, and L2 = o-phenanthroline or 2-(2-pyridyl)benzimidazole, have shown in vitro antiharpes activity toward both HSV types 1 and 2 (HSV-1 and HSV-2) [25]. In addition, a series of mono-, di-, and tri-organotin halides (alkyl and phenyl) exhibited weak antiherpes activity in the same assay system. In a related study [26], this group looked at the same compounds and their activity against both DNA (herpes virus types 1 and 2), a TK (thymidine kinase deficient) strain of HSV type 1, and vaccinia virus. The RNA viruses were vesicular stomatitis virus, coxsackie virus type B4, sindbis virus type 3, and human immunodeficiency virus (HIV). Overall, the complexes exhibited weak antiviral activity and low selectivity. Most of the complexes were active against one or more of the three strains of HSVs. By comparison, only three complexes were active against any of the RNA viruses. None of the compounds were active against vesicular stomatitis or parainfluenza virus or HIV virus.
Future work will examine the ability of these and additional organotin polymers to inhibit virus replication in not only transformed cell lines but also in normal cell lines, a condition that better mimics antiviral therapy of humans. These studies are also consistent with a need to watch the particular protocols employed in making even simple biological tests. While the initial cell concentration greatly influences the end values, they appear, for this case, to have little influence on the general trends.
As with other studies, the dibutyltin product appears to generally offer the best general ability to inhibit viral and cell line growth for the tested species. This is fortunate since of the organotin moieties, the dibutyltin compounds typically offer the least toxicity to humans.
In summary, the organotin polymers derived from diethyltin, dibutyltin, and diphenyltin, in particular, showed good inhibition of both RNA and DNA viruses and are undergoing further testing as antiviral agents in the war against viruses and possible bioterrorism involving viruses. They also exhibit good inhibition of a number of cancer lines. It has been suggested and occasionally demonstrated that relationships do exist between virus infections and cancer [27].
References
H. J. Schaeffer, US Pat. 4199574 (1976, to Wellcome)
Elion G., Furman P., Fyfe J., Miranda P., Beauchamp L., Schaeffer H.J., (1977) Proc. Natl. Acad. Sci. USA 74: 5716
Physicians’ Desk Reference, 55 Edition, Medical Economics (Thompson Healthcare, Montville, NJ, 2001)
Sabir T., Carraher C. (2005) J. Polym. Mater. 22: 449
A. Garcia-Raso, J. Fiol, F. Badenas, R. Cons, A. Terron, and M. Quiros, J. Chem. Soc., Dalton Trans. 167 (1999)
Turel I., Leban I., Gruber K., (1996) J. Inorg. Biochem. 63: 41
C. Carraher, Macromolecules Containing Metal and Metal-Like Elements. Vol. 4. Group IVB Polymers, Chpt. 10 (Wiley, Hoboken, NJ, 2005)
C. Carraher and D. Siegmann-Louda, Macromolecules Containing Metal and Metal-Like Elements. Vol. 3. Biomedical Applications, Chpt. 4 (Wiley, Hoboken, NJ, 2004)
C. Carraher, D. Winter (1971) Makromol. Chem. 141: 237
Carraher C., Scherubel G., (1972) Makromol. Chem. 152: 61
Carraher C., Scherubel G., (1971) J. Polymer Science, A-1 9: 983
C. Carraher and D. Winter, Makromol. Chem. 152, 55 (1972); 141, 259 (1971)
Carraher C., Winter D. (1973) J. Macromol. Sci.-Chem. A7: 1349
Carraher C., (1973) Angew. Makromol. Chem. 31: 115
Freshney R. (1994) Culture of Animal Cells: A Manual of Basic Technique, 3rd Ed. Wiley-Liss, NY
Willard M. (2002) J. Virol. 76: 5220
S. Koyano, T. Suzutani, I. Yoshida, M. Azuma, M. (1996) Antimicrobial Agents and Chemo. 40: 920
C. Carraher, T. Sabir, and C. Carraher. (2006) J. Polym. Mater. 23: 143
Carraher C., Sabir T., Carraher C. (2006) Polym. Mater. Sci. Eng. 94: 553
Ksiazek T., Erdman D., Goldsmith C., Zaki S., Peret T., Emery S., Tong S., Urbani C., Comer J., Lim W., Rollin P., Nghiem K., Dowell S., Ling A., Humphary C., Shieh W., Guarner J., Paddock C., Rota P., Fields B., DeRisi J., Yang J., Cox N., Hughes J., LeDuc J., Bellini W., Anderson L., (2003) N. Engl. J. Med. 348(20): 1967
Drosten C., Gunther S., Preiser W., Van Der Werf S., Brodt H., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R., Berger A., Burguiere A., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H., Osterhaus A., Schmitz H., Doerr H., (2003) N. Eng. J. Med. 348(20): 1953
Cunliffe N., Bresee N., Hart C., (2002) J. Infect. 45(1): 1
Shmulevitz M., Marcato P., Lee P.W., (2005) Oncogene 24: 7720
Picardi A., Gentilucci U., Zardi E., D’Avola E., Amoroso A., Afeltra A., (2004) Curr. Pharm. Des. 10(17): 2081
Ward S., Taylor R., Craig C., Crowe A., (1988) Appl. Organometal. Chem. 2(1): 47
Ward S., Taylor R., Craig C., Crowe A., Balzarini J., De Clercq E., (1989) Appl. Organometal, Chem. 3(5):431
Pagano J.S., Basler M., Buendia M.A., Damania B., Khalili K., Raab-Traub N., Roizman B., (2004) Semin Cancer Biol. 14(6): 453
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carraher, C.E., Sabir, T.S., Roner, M.R. et al. Synthesis of Organotin Polyamine Ethers Containing Acyclovir and their Preliminary Anticancer and Antiviral Activity. J Inorg Organomet Polym 16, 249–257 (2006). https://doi.org/10.1007/s10904-006-9050-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-006-9050-y