Abstract
Retail sales of over the counter (OTC) medications have risen steadily over the years; however, these products are not without potential risk. The aim of this study was to describe the marketing strategies used to promote OTC medicines to children and their parents. Data were collected from 187 product packaging of fever reducer, allergy, and cough medicines posted on the Internet sites of three of the largest pharmacies in the United States. Specific marketing information was collected related to flavorings and pictures appearing on the packaging. There was no significant difference between the type of medicine and whether they were flavored or not, as almost every product (95.7 %) indicated that it resembles a food flavoring. On almost all the packaging (92.5 %) the flavor was also indicated by words in different font sizes. Most of the products (83.4 %) showed a picture of a food product on their packaging. There was a significant difference between the medicine types by picture, with fever medicines having more pictures of food than either cough or allergy medicine. Stronger regulations of marketing strategies of these products are needed.
Similar content being viewed by others
Introduction
According to the Consumer Healthcare Products Association, retail sales of over the counter (OTC) medications have risen steadily over the years, with 2015 figures indicating retail sales of 32.1 billion dollars [1]. Parents and children alike may be drawn to the appeal of the packaging of certain medications, as they often feature pictures of foods and contain colorings flavorings of commonly eaten foods. There is a paucity of research examining the marketing tactics of OTC medicines, but similar strategies have been applied to food and beverages aimed at children [2]. These marketing tactics create the impression that these medicines are food, which can have negative ramifications for overconsumption and put the child at great risk. Such appealing characteristics have been documented in children’s products like vitamins [3, 4] and toothpaste [5, 6].
An average of $338 per year is spent on OTC products in the United States [7]. The majority (85 %) of US parents prefer OTC medicines as a first line of treatment for their children’s minor ailments [7]. On one hand, OTC medicines are seen as a great benefit, resulting in increased economic productivity as a result of fewer days of missed work for parents with ill children [7]; on the other hand, there are dangers that accompany these products. Of all emergency room visits by children under 6 years old from 2010 to 2013 involving a medication, 43.4 % were attributed to an OTC medication [8]. Almost all (91 %) of the cases of OTC liquid medication exposures involved four medications (acetaminophen, cough and cold remedies, ibuprofen, or diphenhydramine) and of these, 87 % involved pediatric formulations [8]. An objective of Healthy People 2020 is to reduce emergency department visits for unintentional pediatric medication overdose [9], thus, it has been suggested that improved safety packaging and education be provided.
OTC cough and cold medications are considered not effective in in acute cough [10] or in treating children with the common cold and may cause serious side effects, which includes death [11]. In 2008, the FDA warned against the use of cough and cold medications in children under the age of four, going as far as to state that use in children under two could result in potentially life threatening side effects [12]. The FDA statement supports actions of manufacturers of OTC cough and cold medicines medicine to modify the labels on their products and create packaging that is more child resistant [12]. They urged consumers to understand that cough and cold medicines do not cure the cough or cold, but rather treat the symptoms [12]. Despite these warnings, one study found that use of these medications was still commonplace [13]. Reasons for this may include difficulty understanding the indications as well as packaging label characteristics [14]. Graphics on the front panel of the product, have been shown to influence caregivers’ perceptions of the appropriateness of OTC cough and cold medicine for young children [14, 15].
Given the attention to the labeling of OTC medicines, and the potential for misuse due to marketing practice that mirror those used in marketing food and beverages to children [16], the aim of this study was to describe the marketing practices used to promote OTC medicines to children and their parents. This study was exempt from IRB, as it did not involve human subjects.
Methods
Internet sites from three of the largest United States based chain pharmacies [17] were searched initially in 2015 and then checked and updated for additions in 2016 to garner the sample of infants and children’s OTC medicines. The individual URLs for each product were included in a database and as new products were identified they were added to the running list. Observation involved using the picture of the packaging posted on the pharmacy website as well as noting if there was a tab section which led to details of ingredients and warnings listed along with this product. Inclusion criteria were the following: (1) the product was intended for infants and children, (2) the product was either a fever/pain reducer, allergy relief, or cough relief, (3) the product was available for purchase on at least one pharmacy website.
Data were collected on information from the product packaging posted to the pharmacy website. Specific marketing information was collected related to flavorings and pictures appearing on the bottles. Information related to the medicine included: (1) brand, (2) form of medication, (3) indication of it being dye free or not, (4) amount per dosage, (5) dosage per bottle, (6) presence of warnings, (7) ingredients. Font size was determined using a comparative chart that has been used in previous studies [4]. Two researchers coded 10 % of the products to determine inter-rater reliability. The inter-rater reliability was established at 99.2 %.
Analysis
The means, standard deviations, and frequencies were calculated to describe the number and content of the packaging descriptions. Chi squared analysis was used to compare the number of packaging descriptions by over the counter product type. Statistical significance was set at 0.05. The statistical program used for data analysis was SPSS 23.0.
Results
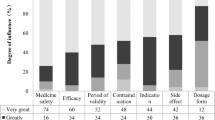
There were 187 products reviewed, of which 33.7 % were fever reducer products, 24.6 % were allergy, and 41.7 % were cough medicines. The majority of these OTC (85.6 %) were in liquid form and the rest (14.4 %) tablets. There was a significant difference between the medicine types by form, either liquid or tablet X 2(2) = 15.91, p < 0.01, with almost all cough medicines (97.4 %) being in liquid form compared to fever medicines (79.4 %) and allergy medicine (73.9 %). As seen in Table 1, less than a third (28.9 %) of the products state that they are dye free on the label, with no significant difference between the three products.
Flavor
There was no significant difference between the products on whether they were flavored or not, as almost every product (95.7 %) indicated that it resembles a food flavoring. The majority (84.9 %) of flavors were fruit (e.g., grape, berry, cherry, etc.) and others as follows: a bubblegum flavor (10.7 %), chocolate (2.2 %) or honey (2.2 %). As seen in Table 1, there was a significant difference between the products in terms of types of flavoring X 2(14) = 38.01, p < 0.01. Every fever medicine product (100.0 %) stated it was flavored, either by some type of fruit flavored (77.8 %) or else bubblegum flavored (22.2 %). Similarly, almost every allergy medicine (95.7 %) stated it was flavored with either fruit (87 %) or bubblegum (13.0 %). The majority (92.3 %) of cough medicines were flavored either by fruit (88.8 %), honey (5.6 %), or chocolate (5.6 %), and none (0.0 %) listed bubblegum flavor.
Font
On almost all the packaging (92.5 %), the flavor was indicated by words in different font sizes. Although, there was a significant difference between the products, X 2(2) = 12.77, p < 0.01, with 100.0 % of fever medications, 95.7 % of allergy medications, and 84.6 % of cough medications having words to indicate the flavor. There was also a significant difference between the products in terms of font sizes X 2(4) = 20.28, p < 0.001, with 25.4 % of fever medications having large a font size, compared to 9.1 % of cough medications and 4.5 % of allergy medications.
Pictures
Most of the products (83.4 %) showed a picture of a food product on their packaging. Of these pictures, most were of fruit (86.6 %), bubblegum (11.2 %), or honey (2.2 %). There was a significant difference between the medicine types by picture, with fever medicines having more pictures of food than either cough or allergy medicine, X 2(6) = 45.40, p < 0.001. Every fever medicine product (100.0 %) listed either a picture of fruit (77.8 %) or bubblegum (22.2 %) on their packaging, while 67.9 % of cough medicines listed either a picture of fruit (64.1 %) or honey (3.8 %) on their packaging, and 87 % of allergy medicines listed either a picture of fruit (78.3 %) or bubblegum (8.7 %) on their packaging.
Discussion
To our knowledge, this is the first study to report on the characteristics of packaging of OTC medicines for children. The packaging of OTC suggests, with pictures of food and font size advertising, that these medicines are food-like with regard to taste. This is concerning given potentially overconsumption and safety of these products. Recent marketing efforts have been aimed at young children, even toddlers, with the use of characters shown to be a popular marketing tactic [18]. While licensed characters were not noted in this study, it was common to find use of food pictures on the OTC medicine. This is of concern since children may mistake this medicine as a food product which can contribute to overdose.
While some of the products reviewed were marketed as being dye free, the majority contained dye. Kobylewski and Jacobson [19] indicate that dyes have toxicological risks and most often serve no purpose other than one that is cosmetic in that it makes a product more appealing. This is a strategy that makes for a product with bold colors that often appeals to consumers [19]. Several studies have pointed to improvements when children consume diets free of artificial food dye [20–22], yet the findings from this study indicate dyes are still common in OTC medicine.
Flavorings were another component that was found in almost every product in this sample of children’s OTC medications. The flavoring resembled some type of food, either fruit, bubblegum, chocolate, or honey. In a study of pediatric oral liquid medications, it was determined that sucrose was a commonly added flavoring which was widely varied in the amount used [23]. In another study focused on label accuracy and pharmaceutical excipients, findings lead to the conclusions that consumers may be exposed more to flavorings like sugar and aspartame than indicated on the label due to inaccuracies on labels [24].
Most of packaging included pictures and words in different fonts sizes to indicate what the flavor was. Lokker et al. [14] found that when caregivers were allowed to view only the front of OTC pediatric cold medicine packaging, 86 % of the time they misinterpreted the product and thought that it was appropriate for children under 2 years old. This may be even more concerning for consumers who visit internet sites and only see the front of the package displayed, having to use additional navigational features or tabs to find further details on ingredients, directions, and warnings.
Recent research suggests that adolescents are intentionally misusing OTC medication and presenting to emergency departments with a wide range of disturbing symptoms [13]. In an empirical review of 53 studies on the topic, researchers found that OTC medication abuse is an international problem that has not been well understood [25]. It is suggested that parents and caregivers receive training in proper use of OTC medications to avoid harmful effects like overdose [26], and abuse. Even small steps like adding pictorial aids have been shown to help [15].
This study is limited by the cross-sectional design, and the fluctuating nature of products for sale on the Internet. Despite these limitations, this research offers insight into an important issue, the exposure to marketing tactics of children’s OTC medicines when purchasing these types of medications over the Internet. Stronger regulations of marketing strategies of these products are needed.
References
Consumer Healthcare Products Association. (2016). OTC Retail Sales 1964–2015. http://www.chpa.org/OTCRetailSales.aspx.
Mehta, K., Phillips, C., Ward, P., et al. (2012). Marketing foods to children through product packaging: Prolific, unhealthy and misleading. Public Health Nutrition, 15(9), 1763–1770.
Basch, C. H., Roberts, K., Ethan, D., & Samayoa-Kozlowsky, S. (2014). An examination of marketing techniques used to promote children’s vitamins in parenting magazines. Global Journal of Health Science., 7(3), 171–176.
Ethan, D., Basch, C. H., Samuel, L., Quinn, C., & Dunne, S. E. (2015). An examination of product packaging marketing strategies used to promote pediatric vitamins. Journal of Community Health, 40(3), 564–568.
Basch, C. H., & Rajan, S. (2014). Marketing strategies and warning labels on children’s toothpaste. Journal of Dental Hygiene, 88(5), 312–315.
Basch, C. H., Hammond, R., Guinta, A., Rajan, S., & Basch, C. E. (2013). Advertising of toothpaste in parenting magazines. Journal of Community Health, 38(5), 911–914.
Consumer Healthcare Products Association. (2016). Statistics on OTC Mediation. http://www.chpa.org/marketstats.aspx.
Lovegrove, M. C., Weidle, D. S., & Budnitz, D. S. (2015). Trends in emergency department visits for unsupervised pediatric medication exposures, 2004–2013. Pediatrics,. doi:10.1542/peds.2015-2092.
Healthy People.gov. Healthy People 2020: MPS-5.4 Reduce emergency department (ED) visits for medication overdoses among children less than 5 years of age https://www.healthypeople.gov/2020/topics-objectives/objective/mps-54.
Shefrin, A. E., & Goldman, R. D. (2009). Use of over-the-counter cough and cold medications in children. Canadian Family Physician, 55(11), 1081–1083.
Smith, S. M., Schroeder, K., & Fahey, T. (2012). Over-the-counter medications for acute cough in children and adults in ambulatory settings. The Cochrane Database of Systematic Reviews,. doi:10.1002/14651858.
U.S. Food and Drug Administration. Using over-the-counter cough and cold products in children. FDA Consumer Health Information Website. http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/ucm048524.pdf.
Finkelstein,Y., Goel, G., Hutson, J.R., Armstrong, J., Baum, C.R., Wax, P., Brent, J., Toxicology Investigators Consortium (ToxIC). (2015). Drug misuse in adolescents presenting to the emergency room. Pediatric Emergency Care. PMID:26466148.
Lokker, N., Sanders, L., Perrin, E. M., et al. (2009). Parental misinterpretations of over-the-counter pediatric cough and cold medicine. Pediatrics, 123(6), 1464–1471.
Chan, H. K., Hassali, M. A., Lim, C. J., Saleem, F., & Tan, W. L. (2015). Using pictograms to assist caregivers in liquid medication administration: A systematic review. Journal of Clinical Pharmacy and Therapeutics, 40, 266–272.
Story, M., & French, S. (2004). Food advertising and marketing directed at children and adolescents in the US. International Journal of Behavioral Nutrition and Physical Activity, 1(3), 1–17.
SK&A. (2016). National pharmacy market summary: Market insights report. S K & A Website. http://www.skainfo.com/reports/most-powerful-pharmacies.
Roberto, C. A., Baik, J., Harris, J. L., et al. (2010). Influence of licensed characters on children’s taste and snack preferences. Pediatrics, 126, 88–93.
Kobylewski, S., Jacobson, M. F. (2010). Food dyes: A rainbow of risks. Center for Science in Public Interest. https://cspinet.org/new/pdf/food-dyes-rainbow-of-risks.pdf.
Stevens, L. J., Kuczek, T., Burgess, J. R., et al. (2011). Dietary sensitivities and ADHD symptoms: Thirty-five years of research. Clinical Pediatrics, 50(4), 279–293.
Stevenson, J., Buitelaar, J., Cortese, S., et al. (2014). Research review: The role of diet in the treatment of attention-deficit/hyperactivity disorder—An appraisal of the evidence on efficacy and recommendations on the design of future studies. Journal of Child Psychology and Psychiatry and Allied Disciplines, 55(5), 416–427.
Nigg, J. T., & Holton, K. (2014). Restriction and elimination diets in ADHD treatment. Child and Adolescent Psychiatric Clinics of North America, 23(4), 936–953.
Hill, E. M., Flaitz, C. M., & Frost, G. R. (1988). Sweetener content of common pediatric oral liquid medications. American Journal of Hospital Pharmacy, 45(1), 135–142.
Balbani, A. P., Stelzer, L. B., & Montovani, J. C. (2006). Pharmaceutical excipients and the information on drug labels. Brazilian Journal of Otorhinolaryngology, 72(3), 400–406.
Cooper, R. J. (2013). Over-the-counter medicine abuse—A review of the literature. Journal of Substance Use, 18(2), 82–107.
Karpa, K. D., Felix, T. M., & Lewis, P. R. (2015). Adverse effects of common drugs: Children and adolescents. FP Essentials, 436, 17–22.
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Basch, C.H., Roberts, K.J., Zagnit, E.A. et al. Marketing Strategies Used to Promote Children’s Medicine Sold on Internet Sites of Pharmaceutical Stores. J Community Health 41, 1212–1216 (2016). https://doi.org/10.1007/s10900-016-0205-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-016-0205-7