Abstract
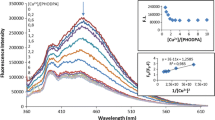
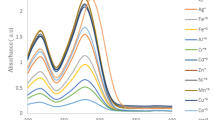
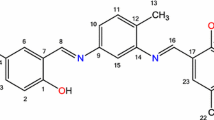
Four new Schiff base ligands carrying naphthalene groups were prepared from the reaction of 2,4-diamino-6-methyl-1,3,5-triazine and 2,4-diamino-6-undecyl-1,3,5-triazine with 2-hydroxy-1-naphthaldehyde. The influence of a series of metal ions including Cu2+, Co2+, Hg2+, Al3+, Cr3+, Fe3+, Pb2+, Ni2+, Cd2+, Zn2+, Mn2+, Ag+, Ba2+, Ca2+ and Mg2+ on the spectroscopic properties of the ligands was investigated by means of absorption and emission spectrometry. The results of spectrophotometric and spectrofluorimetric titrations disclosed the complexation stoichiometry and complex stability constant of the ligands with metal ions. A simple spectrofluorimetric method was developed using the Schiff base derived from 2,4-diamino-6-undecyl-1,3,5-triazine to determine Hg2+ ion. No cleanup or enrichment of the tap water sample was required. A modified standard addition method was used to eliminate matrix effect. The standard addition graph was linear between 0.2 and 2.6 mg/L in determination of Hg2+. Detection and quantification limits were 0.08 and 0.23 mg/L, respectively. The simple and cost-effective method can be applied to water samples.










Similar content being viewed by others
References
Etaiw SEH, Abd El-Aziz DM, Abd El-Zaher EH, Ali EA (2011) Synthesis, spectral, antimicrobial and antitumor assessment of Schiff base derived from 2-aminobenzothiazole and its transition metal complexes. Spectrochim Acta - Part A Mol Biomol Spectrosc 79:1331–1337. doi:10.1016/j.saa.2011.04.064
Golcu A, Tumer M, Demirelli H, Wheatley RA (2005) Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity. Inorg Chim Acta 358:1785–1797. doi:10.1016/j.ica.2004.11.026
Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel JM (2007) Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules 12:1720–1730. doi:10.3390/12081720
Nawaz H, Akhter Z, Yameen S, Siddiqi HM, Mirza B, Rifat A (2009) Synthesis and biological evaluations of some Schiff-base esters of ferrocenyl aniline and simple aniline. J Organomet Chem 694:2198–2203. doi:10.1016/j.jorganchem.2009.02.032
Priya NP, Arunachalam S, Manimaran A, Muthupriya D, Jayabalakrishnan C (2009) Mononuclear Ru(III) Schiff base complexes: Synthesis, spectral, redox, catalytic and biological activity studies. Spectrochim Acta - Part A Mol Biomol Spectrosc 72:670–676. doi:10.1016/j.saa.2008.10.028
Afkhami A, Abbasi-Tarighat M, Khanmohammadi H (2009) Simultaneous determination of Co2+, Ni2+, Cu2+ and Zn2+ ions in foodstuffs and vegetables with a new Schiff base using artificial neural networks. Talanta 77:995–1001. doi:10.1016/j.talanta.2008.07.065
Aksuner N, Henden E, Yilmaz I, Cukurovali A (2009) A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a Schiff base. Dyes Pigments 83:211–217. doi:10.1016/j.dyepig.2009.04.012
Kara D, Fisher A, Hill SJ (2009) Determination of trace heavy metals in soil and sediments by atomic spectrometry following preconcentration with Schiff bases on Amberlite XAD-4. J Hazard Mater 165:1165–1169. doi:10.1016/j.jhazmat.2008.10.111
Marahel F, Ghaedi M, Montazerozohori M, Nejati Biyareh M, Nasiri Kokhdan S, Soylak M (2011) Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples. Food Chem Toxicol 49:208–214. doi:10.1016/j.fct.2010.10.018
Bhagat S, Sharma N, Chundawat TS (2013) Synthesis of some salicylaldehyde-based schiff bases in aqueous media. J Chem. Article ID 909217
Yang Z, Sun P (2006) Compare of three ways of synthesis of simple Schiff base. Molbank Article ID M514s
Alias M, Kassum H, Shakir C (2014) Synthesis, physical characterization and biological evaluation of Schiff base M(II) complexes. J Assoc Arab Uni basic. Appl Sci 15:28–34
Sabah HH (2014) Synthesis, spectroscopic characterization of schiff bases derived from 4,4'-methylen di aniline. Der Pharma Chemica 6:38–41
Kulshrestha A, Baluja S (2010) Microwave promoted synthesis of some Schiff bases. Arch Appl Sci Res 2:221–224
Shockravi A, Sadeghpour M, Olyaei (2010) A simple and efficient procedure for the synthesis of symmetrical bis-schiff bases of 5,5′-methylenebis(2-aminothiazole) under solvent-free conditions. Syn Commun 40:2531–2538
Mishra AP, Sharma N, Jain RK (2013) Microwave synthesis, spectral, thermal and antimicrobial studies of some Ni(II) and Cu(II) Schiff base complexes. Open J Synt. Theory Appl 2:56–62
Nikpassand M, Fekri LZ, Sharafi S (2013) An efficient and green synthesis of novel azo Schiff base and its complex under ultrasound irradiation. Orient J Chem 29:1041–1046
Vazquez MA, Landa M, Reyes L, Miranda R, Tamariz J, Delgado F (2004) Infrared irradiation: effective promoter in the formation of n-benzylideneanilines in the absence of solvent. Synth Commun 34:2705–2718
Murhekar MM, Khadsan RE (2011) Synthesis of Schiff bases by organic free solvent method. J Chem Pharm Res 3:846–849
Schmeyers J, Toda F, Boy J, Kaupp G (1998) Quantitative solid–solid synthesis of azomethines. J Chem Soc Perkin Trans 2(4):989–994
Eissa HH (2013) Synthesis and characterization of new azo-schiff bases and study biological activity. J Curr Res Sci 1:96–103
Naeimi H, Nazifi ZS (2013) Convenient and mild synthesis and characterisation of some new. Bull Chem Soc Ethiop 27:143–149
Ali A, Abdullah N, Maah MJ (2013) Synthesis, characterization and antioxidant studies on 4-phenyl-1,3,5-triazine-2,6-diamine schiff bases and their nickel(II), copper(II) and zinc(II) complexes. Asian J Chem 25:3105–3108
Singh RK, Kukrety A, Saxena RC, Thakre GD, Atray N, Ray SS (2016) Novel Triazine Schiff Base-based cationic Gemini surfactants: synthesis and their evaluation as Antiwear, antifriction, and anticorrosive additives in polyol. Ind Eng Chem Res 55:2520–2526
Mobinikhaledi A, Steel PJ, Polson M (2009) Rapid and efficient synthesis of schiff bases catalyzed by copper nitrate. Syn React Inorg Metal-Org Nano-Met Chem 39:189–192
Bourson J, Valeur B (1989) Ion-responsive fluorescent compounds 2 Cation-steered intramolecular charge transfer in a crowned merocyanine. J Phys Chem 93:3871–3876
Başoğlu A, Tosun G, Ocak M, Alp H, Yaylı N, Ocak Ü (2015) Simple time-saving method for iron determination based on fluorescence quenching of an azaflavanon-3-ol compound. J Agric Food Chem 63:2654–2659
Issa RM, Khedr AM, Rizk HF (2008) 1H NMR, IR and UV/VIS spectroscopic studies of some schiff bases derived from 2-aminobenzothiazole and 2-amino-3-hydroxypyridine. J Chin Chem Soc 55:875–884
Salman SR, Kamounah FS (2003) Tautomerism in 1-hydroxy-2-naphthaldehyde Schiff bases: calculation of tautomeric isomers using carbon-13 NMR. Spectroscopy 17:747–752
Ünver H, Kabak M, Zengin DM, Durlu TN (2008) Crystal structure and tautomerism of 1-[N-(4-Iodophenyl)]aminomethylidene-2(1H)naphthalenone, Zeitschrift für Naturforschung B. 56:1003-1008
Hayvali Z, Yardimci D (2008) Synthesis and spectroscopic characterization of asymmetric Schiff bases derived from 4′-formylbenzo-15-crown-5 containing recognition sites for alkali and transition metal guest cations. Transit Met Chem 33:421–429
Sun Y, Wu AT (2010) Indole-based fluorescent sensor for selective detection of Hg(II. J Fluoresc 20:553–540
Li Y, Zhang X, Zhu B, Xue J (2011) A disulfide-linked naphthalimide dimer for Hg(II) detection in aqueous solution. J Fluoresc 21:1343–1348
Loo AYY, Lay YP, Kutty MG, Timpe O, Behrens M (2012) Spectrophotometric determination of mercury with iodide and rhodamine B. Sains Malaysiana 41(2):213–218
Hamzaa A, Bashammakhb AS, Al-Sibaai AA, Al-Saidi HM, El-Shahawi MS (2010) Part 1. Spectrophotometric determination of trace mercury (II) in dental-unit wastewater and fertilizer samples using the novel reagent 6-hydroxy-3-(2- oxoindolin-3-ylideneamino)-2-thioxo-2H-1,3-thiazin-4(3H)-one and the dual-wavelength β-correction spectrophotometry. J Hazard Mater 178:287–292
Wanga J, Huang L, Xue M, Liu L, Wanga Y, Gao L, Zhu J, Zou Z (2008) Developing a novel fluorescence chemosensor by self-assembly of bis-schiff base within the channel of mesoporous sba-15 for sensitive detecting of Hg2+ ions. Appl Surf Sci 254:5329–5335
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1253 kb)
Rights and permissions
About this article
Cite this article
Ocak, M., Ak, T., Aktaş, A. et al. Metal Complexation Properties of Schiff Bases Containing 1,3,5-Triazine Derived from 2-Hydroxy-1-Naphthaldehyde in Solution. A Simple Spectrofluorimetric Method to Determine Mercury (II). J Fluoresc 27, 59–68 (2017). https://doi.org/10.1007/s10895-016-1934-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1934-9