Abstract
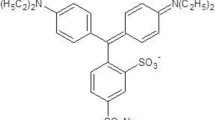
In the present work dual beam thermal lens technique is used for studying the solvent dependency on the quantum efficiency of a novel dye used for biomedical applications. The role of solvent in the absolute fluorescence quantum yield of 4-[(4-Aminophenyl)-(4-imino-1-cyclohexa-2, 5- dienylidene) methyl] aniline hydrochloride is studied using thermal lens technique. It is observed that the variation in solvents and its concentration results considerable variations in the fluorescence quantum yield. These variations are due to the non-radiative relaxation of the absorbed energy and because of the different solvent properties. The highest quantum yield of the dye is observed in the polar protic solvent-water.






Similar content being viewed by others
References
Rurack K, Spieles M (2011) Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600–1000 nm. Anal Chem 83:1232–1242
Ishida H, Tobita S, Hasegawa Y, Katoh R, Nozaki K (2010) Recent advances in instrumentation for absolute emission quantum yield measurements. Coord Chem Rev 254:2449–2458
Wurth C, Grabolle M, Pauli J, Spieles M, Resch-Genger U (2011) Comparison of methods and achievable uncertainties for the relative and absolute measurement of photoluminescence quantum yields. Anal Chem 83:3431–3439
Estupinan-Lopez C, Tolentino Dominguez C, de Araujo RE (2013) Eclipsing thermal lens spectroscopy for fluorescence quantum yield measurement. Opt Express 21:18592
Sulatskaya AI, Maskevich AA, Kuznetsova IM, Uversky VN, Turoverov KK (2010) Fluorescence quantum yield of Thioflavin T in rigid isotropic solution and incorporated into the amyloid fibrils. PLoS ONE 5:e15385
Brannon JH, Magde D (1978) Absolute quantum yield determination by thermal blooming. Fluorescein. J Phys Chem 82:705–709
Shen J, Snook RD (1989) Thermal lens measurement of absolute quantum yields using quenched fluorescent samples as references. Chem Phys Lett 155:583
Resch-Genger U, DeRose PC (2010) Fluorescence standards: classification, terminology, and recommendations on their selection, use, and production (IUPAC Technical Report). Appl Chem 82:2315–2335
BiniPathrose, H. Sahira, V.P.N. Nampoori, P. Radhakrishnan, A. Mujeeb (2014) Variations in fluorescence quantum yield of basic fuchsin with silver nanoparticles prepared by femtosecond laser ablation. Spectrochim Acta A Mol Biomol Spectrosc 128. http://www.sciencedirect.com/science/journal/13861425/128/supp/C:522–526.
Bini P, Nampoori VPN, Radhakrishnan P, Mujeeb A (2014) Measurement of absolute fluoroscence quantum yield of basic fuchsin solution using a dual-beam thermal lens technique. J Fluoresc 24(3):895–898
Joseph SA, Misha H, Mathew S, Sharma G, Soumya VM, Hadiya P, Radhakrishnan VPNN (2010) Thermal diffusivity of rhodamine 6G incorporated in silver nanofluid measured using mode-matched thermal lens technique. Opt Commun 283:313–317
Rowan BC, Wilson LR, Richards BS (2008) Advanced material concepts for luminescent solar concentrators. IEEE J Sel Top Quantum Electron 14(5):1312–1322
Navarro JRG, Plugge M, Loumaigne M, Sanchez-Gonzalez A, Débarre BMA, Brouwer AM, Werts MHV (2010) Probing the interactions between disulfide-based ligands and gold nanoparticles using a functionalized fluorescent perylene-monomide dye. Photochem Photobiol Sci 9:1042–1054
Berezin MY, Achilefu S (2010) Fluorescence lifetime measurements and biological imaging. Chem Rev 110:2641–2684
Kleinert T, Doster W, Leyser H, Petry W, Schwarz V, Settles M (1998) Solvent composition and viscosity effects on the kinetics of co binding to horse myoglobin. Biochemistry 37(2):717–733
Shen J, Lowe RD, Snook RD (1992) A model for CW laser induced mode-mismatched dual-beam thermal lens spectrometry. Chem Phys 165:385–396
Dovichi NJ, Harris JM (1979) Laser induced thermal lens effect for calorimetric trace analysis. Anal Chem 51(6):728–731
Bindhu CV, Harilal SS, Issac RC, Nampoori VPN, Vallabhan CPG (1995) Laser induced thermal lens effect in rhodamine B - signature of resonant two photon absorption. Mod Phys Lett B 9:1471–1477
Twarowski AJ, Kliger DS (1977) Multiphoton absorption spectra using thermal blooming theory. Chem Phys 20:253–258
Bindhu CV, Harilal SS, Varier GK, Issac RC, Nampoori VPN, Vallabhan CPG (1996) Measurement of the absolute fluorescence quantum yield of rhodamine B solution using a dual-beam thermal lens technique. J Phys D Appl Phys 29:1074–1079
Wurth C, Grabolle M, Pauli J, Spieles M, Resch-Genger U (2011) Comparison of methods and achievable uncertainties for the relative and absolute measurement of photoluminescence quantum yields. Anal Chem 83:3431–3439
Shemeena Basheer N, Rajesh Kumar B, Kurian A, George SD (2013) Silver nanoparticle size-dependent measurement of quantum efficiency of Rhodamine 6G. Appl Phys B 113:581–587
Raikar US, Tangod VB, Mastiholi BM, Sreenivasa S (2010) Solvent effects and photophysical studies of ADS560EI laser dye. Afr J Pure Appl Chem 4(9):188–197
Thankappan A, Sheenu T, Nampoori VPN (2013) Solvent effect on the third order optical nonlinearity and optical limiting ability of betanin natural dye extracted from red beetroot. Opt Mater 35:2332–2337
Banuelos Prieto J, Lopez Arbeloa F, Virginia Martınez Martınez T, Lopez A, Lopez Arbeloa I (2004) Photophysical properties of the pyrromethane 597 dye: solvent effect. J Phys Chem A 108:5503–5508
Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA (2005) Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorg Chem 33:415–425
Zehentbauer FM, Moretto C, Stephen R, Thevar T, Gilchrist JR, Pokrajac D, Richard KL, Kiefer J (2014) Fluorescence spectroscopy of Rhodamine 6G: concentration and solvent effects. Spectrochim Acta A Mol Biomol Spectrosc 121:147–151
Bodke YD, Shankerrao S, Harishkumar HN (2013) Synthesis of 2-(1-Benzofuran-2-yl)-4-(1, 3-benzoxazol-2-yl/ 1, 3- benzothiazol-2-yl) quinolines as blue green fluorescent probes. J Chem 794810:7
Lakowicz JR (2006) Principles of fluorescence spectroscopy. New York
Ghosh R, Palit DK (2013) Dynamics of solvent controlled excited state intramolecular proton transfer coupled charge transfer reactions. Photochem Photobiol Sci 12(6):987–995
Nordin MN, Li J, Clowes SK, Curry RJ (2012) Temperature dependent optical properties of PbS nanocrystals. Nanotechnology 23:275701
Sasaki C, Naito H, Iwata M, Kudo H, Yamada Y, Taguchi T, Jyouichi T, Okagawa H, Tadatomo K, Tanaka H (2003) Temperature-independent stokes shift in an InxGa1-x N epitaxial layer revealed by photoluminescence excitation spectroscopy. J Appl Phys 93:1642
Stsiapura VI, Maskevich AA, Kuzmitsky VA, Uversky VN, Kuznetsova IM, Turoverov KK (2008) Thioflavin T as a molecular rotor: fluorescent properties of thioflavin T in solvents with different velocity. J Phys Chem B 112(49):15893–15902
Acknowledgments
The author is grateful to the University Grants Commission, New Delhi for the research fellowships. DST is also acknowledged for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pathrose, B., Nampoori, V.P.N., Radhakrishnan, P. et al. Solvent Dependency in the Quantum Efficiency of 4-[(4-Aminophenyl)-(4-imino-1-cyclohexa-2, 5- dienylidene) methyl] Aniline Hydrochloride. J Fluoresc 25, 739–744 (2015). https://doi.org/10.1007/s10895-015-1560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1560-y