Abstract
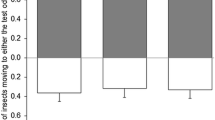
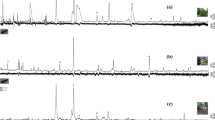
Solid-phase microextraction (SPME) and gas chromatography coupled with electroantennographic detection (GC-EAD) were used to identify volatile compounds from shoots of riverbank grape (Vitis riparia) that attract the female grape berry moth (GBM, Paralobesia viteana). Consistent EAD activity was obtained for 11 chemicals: (Z)-3-hexen-1-yl acetate, (E)-linalool oxide, (Z)-linalool oxide, nonanal, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene, methyl salicylate, decanal, β-caryophyllene, germacrene-D, and α-farnesene. In flight-tunnel tests that involved female GBM and rubber septa loaded with subsets of these 11 compounds, we found that both the 11-component blend and a seven-component blend, composed of (E)-linalool oxide, (Z)-linalool oxide, nonanal, (E)-4,8-dimethyl-1,3,7-nonatriene, decanal, β-caryophyllene and germacrene-D, elicited equivalent levels of upwind flight as freshly cut grape shoots. The removal of any of the seven compounds from the seven-component blend resulted in a significant decrease in female upwind flight responses. In a field trial with these two synthetic blends, traps equipped with either blend captured more female GBM compared to traps baited with hexane only (control), although the number of females caught was generally low. There were no differences in the number of males captured among treatments. Although in flight-tunnel trials, moths readily flew upwind to both grape shoots and rubber septa loaded with the best lures, they landed on shoots but not on rubber septa. Coupled with relatively low field catches, this suggests that additional host finding cues need to be identified to improve trap efficacy.




Similar content being viewed by others
References
Angioy, A. M., Desogus, A., Barbarossa, I. T., Anderson, P., and Hansson, B. S. 2003. Extreme sensitivity in an olfactory system. Chem. Sens. 28:279–284.
Ansebo, L., Coracini, M. D. A., Bengtsson, M., Liblikas, I., Ramirez, M., Borg-Karlson, A. K., Tasin, M., and Witzgall, P. 2004. Antennal and behavioural response of codling moth Cydia pomonella to plant volatiles. J. Appl. Entomol. 128:488–493.
Bernays, E. A., and Chamman, R. F. 1994. Host plant selection by phytophagous insects. Chapman & Hall, New York.
Blackmer, J. L., and Cañas, L. A. 2005. Visual cues enhance the response of Lygus hesperus (Heteroptera: Miridae) to volatiles from host plants. Environ. Entomol. 34:1524–1533.
Blackmer, J. L., Rodriguez-Saona, C., Byers, J. A., Shope, K. L., and Smith, J. P. 2004. Behavioral response of Lygus hesperus to conspecifics and headspace volatiles of alfalfa in a Y-tube olfactometer. J. Chem. Ecol. 30:1547–1564.
Bruce, T. J. A., Wadhams, L. J., and Woodcock, C. M. 2005. Insect host location: a volatile situation. Trend. Plant Sci. 10:269–274.
Butler, L. I., and Mcdonough, L. M. 1979. Insect sex-pheromones—evaporation rates of acetates from natural-rubber septa. J. Chem. Ecol. 5:825–837.
Cha, D. H., Hesler, S. P., Moser, C. L., Nojima, S., Linn, C. E., Roelofs, W. L., and Loeb, G. M. 2008. Flight tunnel responses of female grape berry moth (Paralobesia viteana) to host plants. J. Chem. Ecol.
Clark, L. G., and Dennehy, T. J. 1988. Oviposition behavior of grape berry moth. Entomol. Exp. Appl. 47:223–230.
Coracini, M., Bengtsson, M., Liblikas, I., and Witzgall, P. 2004. Attraction of codling moth males to apple volatiles. Entomol. Exp. Appl. 110:1–10.
Davies, E. 1987. Wound responses in plants, pp, pp. 243–264, in D. Davies (ed.). The Biochemistry of PlantsAcademic, London.
Dozier, H. L., and Butler, H. G. 1929. Life history and control of the grape berry moth in Delaware. J. Econom. Entomol. 22:132–136.
Engelberth, J., Alborn, H. T., Schmelz, E. A., and Tumlinson, J. H. 2004. Airborne signals prime plants against insect herbivore attack. PNAS USA 101:1781–1785.
Ephrussi, B., and Beadle, G. W. 1936. A technique for transplantation of Drosophila. Am. Nat. 70:218–225.
Gleissner, B. D. 1943. Biology and control of berry moth in the Erie grape belt. Pennsylvania State College School of Agriculture Bulletin 451:1–74.
Goodwin, W. H. 1916. The grape berry moth. Ohio Agricultural Experiment Station Bulletin 293:259–307.
Greenwald, R., Chaykovsky, M., and Corey, E. J. 1963. TheWittig reaction using methylsulfinyl carbanion–dimethyl sulfoxide. J. Org. Chem. 28:1128–1129.
Hammack, L. 2003. Volatile semiochemical impact on trapping and distribution in maize of northern and western corn rootworm beetles (Coleoptera: Chrysomelidae). Agri. For. Entomol. 5:113–122.
Heath, R. R., Teal, P. E. A., Tumlinson, J. H., and Mengelkoch, L. J. 1986. Prediction of release ratios of multicomponent pheromones from rubber septa. J. Chem. Ecol. 12:2133–2143.
Hern, A., and Dorn, S. 2004. A female-specific attractant for the codling moth, Cydia pomonella, from apple fruit volatiles. Naturwissenschaften 91:77–80.
Hilker, M., and McNeil, J. 2008. Chemical and behavioral ecology in insect parasitoids: how to behave optimally in a complex odourous environment, pp. 92–112, in E. Wajnberg, C. Bernstein, and J. van Alphen (eds.). Behavioral Ecology of Insect ParasitoidsBlackwell, Malden, MA.
Hoffman, C. J. 1990. Development and validation of a risk assessment program for the management of grape berry moth, Endopiza viteana (Clemens), in New York state. PhD Dissertation, Cornell University, Ithaca, NY.
Leskey, T. C., Zhang, A. J., and Herzog, M. 2005. Nonfruiting host tree volatile blends: novel attractants for the Plum curculio (Coleoptera: Curculionidae). Environ. Entomol. 34:785–793.
Masante-Roca, I., Anton, S., Delbac, L., Dufour, M.-C., and Gadenne, C. 2007. Attraction of the grapevine moth to host and non-host plant parts in the wind tunnel: effects of plant phenology, sex, and mating status. Entomol. Exp. Appl. 122:239–245.
Mitchell, V. J., Manning, L.- A. , Cole, L., Suckling, D. M., and El-Sayed, A. M. 2008. Efficacy of the pear ester as a monitoring tool for codling moth Cydia pomonella (Lepidoptera: Tortricidae) in New Zealand apple orchards. Pest Manage. Sci. 64:209–214.
Mumm, R., and Hilker, M. 2005. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem. Sens. 30:337–343.
Nagarkatti, S., Muza, A., and Saunders, M. 2000. Meridic diet for Endopiza viteana (Lepidoptera: Tortricidae). Can. Entomol. 132:259–261.
Natale, D., Mattiacci, L., Pasqualini, E., and Dorn, S. 2004. Apple and peach fruit volatiles and the apple constituent butyl hexanoate attract female oriental fruit moth, Cydia molesta, in the laboratory. J. Appl. Entomol. 128:22–27.
Nojima, S., Linn, C., Morris, B., Zhang, A. J., and Roelofs, W. 2003. Identification of host fruit volatiles from hawthorn (Crataegus Spp.) attractive to hawthorn-origin Rhagoletis pomonella flies. J. Chem. Ecol. 29:321–336.
Pinero, J. C., Jacome, I., Vargas, R., and Prokopy, R. J. 2006. Response of female melon fly, Bactrocera cucurbitae, to host-associated visual and olfactory stimuli. Entomol. Exp. Appl. 121:261–269.
Rhodes, J. D., Thain, J. F., and Wildon, D. C. 1999. Evidence for physically distinct systemic signalling pathways in the wounded tomato plant. Ann. Bot. 84:109–116.
Roelofs, W. L., Tette, J. P., Taschenberg, E. F., and Comeau, A. 1971. Sex pheromone of the grape berry moth: identification by classical and electroantennogram methods, and field tests. J. Insect Physiol. 17:2235–2243.
SAS Institute. 2000. SAS/STAT User's Guide. SAS Institute, Cary, North Carolina, USA.
SAS Institute. 2006. The GLIMMIX Procedure. <http://www.sas.com>.
Schoonhoven, L. M., Jermy, T., and van Loon, J. J. A. 1998. Insect–Plant Biology. Chapman & Hall, London, UK.
Slingerland, M. V. 1904. The grape berry moth. Cornell University, Agricultural experiment station of the college of agriculture Bulletin 223:43–59.
Taschenberg, E. F. 1945. The biology and control of the grape berry moth Polychrosis vineana (Clemens). PhD Dissertation, Cornell University, Ithaca, NY.
Tasin, M., Anfora, G., Ioriatti, C., Carlin, S., De Cristofaro, A., Schmidt, S., Bengtsson, M., Versini, G., and Witzgall, P. 2005. Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J. Chem. Ecol. 31:77–87.
Tasin, M., Backman, A. C., Bengtsson, M., Ioriatti, C., and Witzgall, P. 2006a. Essential host plant cues in the grapevine moth. Naturwissenschaften 93:141–144.
Tasin, M., Backman, A. C., Bengtsson, M., Varela, N., Ioriatti, C., and Witzgall, P. 2006b. Wind tunnel attraction of grapevine moth females, Lobesia botrana, to natural and artificial grape odour. Chemoecology 16:87–92.
Tasin, M., Backman, A.-C., Coracini, M., Casado, D., Ioriatti, C., and Witzgall, P. 2007. Synergism and redundancy in a plant volatile blend attracting grapevine moth females. Phytochemistry 68:203–209.
Thom, C., Guerenstein, P. G., Mechaber, W. L., and Hildebrand, J. G. 2004. Floral CO2 reveals flower profitability to moths. J. Chem. Ecol. 30:1285–1288.
Vallat, A., and Dorn, S. 2005. Changes in volatile emissions from apple trees and associated response of adult female codling moths over the fruit-growing season. J. Ag. Food Chem. 53:4083–4090.
Visser, J. H. 1986. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 31:121–144.
Wallace, E. K., Albert, P. J., and Mcneil, J. N. 2004. Oviposition behavior of the eastern spruce budworm Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae). J. Insect Behavior 17:145–154.
Weigle, T., Bixby, J., and English-Loeb, G. 1999. Reexamination of grape berry moth management practices in the Lake Erie region. 1998 New York State Fruit Project Reports Relating to IPM. NYS IPM Publication #216. Cornell University Cooperative Extension.
Zhang, A. J., Linn, C., Wright, S., Prokopy, R., Reissig, W., and Roelofs, W. 1999. Identification of a new blend of apple volatiles attractive to the apple maggot, Rhagoletis pomonella. J. Chem. Ecol. 25:1221–1232.
Zhang, A., Oliver, J. E., Aldrich, J. R., Wang, B., and Mastro, V. C. 2002. Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Z. Naturforsch. 57c:553–558.
Acknowledgments
We thank Sara Villani, Charles Moser, Eric Smith, Shinyoung Park, Rachel Tucker, Mike Colizzi, Jessica Worden, Arianna Waheed, and Kevin Conley, for support on various aspects of this research, but particularly their efforts in maintaining the GBM colony and setting up mating cohorts. The manuscript was improved by comments from two anonymous reviewers and the subject editor for which we are grateful. We thank Aijun Zhang and Peter Teal for the gift of the (E)-4,8-dimethyl-1,3,7-nonatriene and germacrene-D, respectively. Authors also thank Arthur Agnello for plastic sheet trap design. Paul Robbins and Shannon Olsson provided valuable technical insights in the beginning of this study. Special thanks go to David Wise at Cornell University Glass Workshop for constructing volatile collection chamber and Jeff and June Pendleton for permitting us to perform field trial in their commercial vineyard. This research was supported by USDA NRI grant #2005-35302-16154 and USDA Viticultural Consortium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cha, D.H., Nojima, S., Hesler, S.P. et al. Identification and Field Evaluation of Grape Shoot Volatiles Attractive to Female Grape Berry Moth (Paralobesia viteana). J Chem Ecol 34, 1180–1189 (2008). https://doi.org/10.1007/s10886-008-9517-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9517-0